Summary
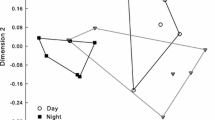
Milkweed (Asclepias syriaca L.: Asclepiadaceae) flowers exposed only to diurnal pollinators (primarily bumble bees, Bombus spp.) matured about eight times as many pods as did flowers exposed only to nocturnal visitors (moths). Rates of pollinaria removal and pollinia insertion were similarly higher during the day than at night. Moths deposited a much lower proportion of their pollinia in stigmatic chambers (one-fifth) than did diurnal pollinators. In spite of the poor service provided by nocturnal pollinators, flowers produced four times as much nectar at night as at day, and sugar production (sucrose equivalents) at night was twice that of the daytime.
Supplemental hand pollinations doubled the production of mature pods, indicating normal pollinia limitation. Mature pod production of clones within a 5-km diameter varied between 0.1 and 3.5 per flowering stem, and numbers of pollinia vectors at these clones were closely correlated with numbers of mature pods produced.
Pollinia-limited clones should thus attract both diurnal and nocturnal pollinators, if costs are not too extreme. Pollinia-limitation should decrease selection for pollinator specialization, especially if the relative importance of different pollinators varies over time, as in this study.
Similar content being viewed by others
References
Bertin RI, Willson MF (1980) Effectiveness of diurnal and nocturnal pollination of two milkweeds. Can J Bot 58:1744–1746
Bierzychudek P (1981) Pollinator limitation of plant reproductive effort. Amer Natur 117:838–840
Bolten AB, Feinsinger P, Baker HG, Baker I (1979) On the calculation of sugar concentration in flower nectar. Oecologia (Berl) 41:301–304
Corbet SA (1978) Bee visits and the nectar of Echium vulgare L. and Sinapis alba L. Ecol Ent 3:25–37
Day GM (1953) The Indian as an ecological factor in the northeastern forest. Ecology 34:329–346
Fahey TJ, Reiners WA (1981) Fire in the forests of Maine and New Hampshire. Bull Torrey Bot Club 108:363–373
Free JB (1970) Insect pollination of crops. Academic Press, New York
Fritz RS, Morse DH (1981) Nectar parasitism of Asclepias syriaca by ants: effect on nectar levels, pollinia insertion, pollinaria removal and pod production. Oecologia (Berlin) 50:316–319
Haber WA, Frankie GW (1982) Pollination of Luehea (Tiliaceae) in Costa Rican deciduous forest. Ecology 63:1740–1750
Levin DA, Kerster HW (1974) Gene flow in seed plants. Evol Biol 7:139–220
Lynch SP (1977) The floral ecology of Asclepias solanoana Woods. Madroño 24:159–177
Macior LW (1965) Insect adaptation and behavior in Asclepias pollination. Bull Torrey Bot Club 92:114–126
Morse DH (1981) Modification of bumble bee foraging: the effect of milkweed pollinia. Ecology 62:89–97
Morse DH (1982) The turnover of milkweed pollinia on bumble bees, and implications for outcrossing. Oecologia (Berlin) 52:187–196
Pellett FC (1976) American honey plants, 5th ed. Dadant and Sons Hamilton Illinois
Proctor M, Yeo P (1973) The pollination of flowers. Collins London
Pyke GH, Waser NM (1981) The production of dilute nectars by hummingbird and honeyeater flowers. Biotropica 13:260–270
Stephenson AG (1981) Flower and fruit abortion: proximate causes and ultimate functions. Ann Rev Ecol Syst 12:253–280
Thomson JD, Plowright RC (1980) Pollen carryover, nectar rewards, and pollinator behavior with special reference to Diervilla lonicera. Oecologia (Berlin) 46:68–74
Waser NM, Price MV (1983) Optimal and actual outcrossing in plants, and the nature of plant-pollinator interaction. In: Jones EC, Little RJ (eds) Handbook of experimental pollination ecology. Van Nostrand Reinhold New York (in press)
Willson MF, Bertin RI (1979) Flower-visitors, nectar production, and inflorescence size of Asclepias syriaca Can J Bot 57:1380–1388
Willson MF, Price PW (1977) The evolution of inflorescence size in Asclepias (Asclepiadaceae). Evolution 31:495–511
Willson MF, Price PW (1980) Resource limitation of fruit and seed production in some Asclepias species. Can J Bot 58:2229–2233
Willson MF, Bertin RI, Price PW (1979) Nectar production and flower visitors of Asclepias verticillata. Amer Midl Natur 102:23–35
Woodson RE (1941) The North American Asclepiadaceae. I. Perspective of the genera. Ann Missouri Bot Gard 28:193–244
Woodson RE (1954) The North American species of Asclepias. Ann Missouri Bot Gard 41:1–211
Wyatt R (1976) Pollination and fruit-set in Asclepias: a reappraisal. Amer J Bot 63:845–851
Wyatt R (1980) The reproductive biology of Asclepias tuberosa: I. Flower number, arrangement, and fruit-set. New Phyt 85:119–131
Wyatt R (1982) Inflorescence architecture: how flower number, arrangement, and phenology affect pollination and fruit-set. Amer J Bot 69:585–594
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morse, D.H., Fritz, R.S. Contributions of diurnal and nocturnal insects to the pollination of common milkweed (Asclepias syriaca L.) in a pollen-limited system. Oecologia 60, 190–197 (1983). https://doi.org/10.1007/BF00379521
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00379521