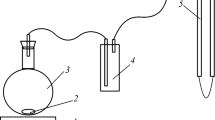
Abstract
A constant composition method has been used for the study of the kinetics of calcium carbonate (calcite) dissolution in which the activities of ionic species in relatively undersaturated solutions are maintained constant. Over a range of relative undersaturation (0.032–0.158) the dissolution reaction appears to be controlled by a bulk diffusion process. The suggestion of a predominantly diffusion-controlled process was supported by the observed low activation energy (2.09 kcal mol−1). The influence of a number of metal ions on the reaction rate has been investigated. The retardation effect of these additives has been attributed to the blocking of active sites by adsorption of metal ions at the crystal surfaces. Inhibition of dissolution by metal ions can be interpreted in terms of a Langmuir isotherm.
Similar content being viewed by others
References
P. E. Cloud, in “Chemical Oceanograph”, Vol. 2, edited by J. P. Diley and G. Skirrow (Academic Press, N.Y., 1965).
K. S. Spiegler, “Salt Water Purification” (Wiley, N.Y., 1962).
M. N. Elliot, Desalination 6 (1969) 87; 8 (1970) 22.
G. H. Nancollas, A. E. Eralp and J. S. Gill, J. Pet. Eng. 18 (1978) 133.
W. H. Taft, in “Development in Sedimentology”, Vol. 9B, edited by G. V. Chilingar, M. J. Bissel and R. W. Fairbridge (Elsevier, 1967), p. 151.
Y. Kitano, Bull. Chem. Soc. Japan 35 (1965) 1973.
J. L. Bischoff and W. S. Fyfe, Amer. J. Sci. 266 (1968) 65.
Berner, A. Robert, ibid. 9 (1978) 278.
P. Koutsou Kos, Z. Amjad, M. B. Tomoson and G. H. Nancollas, J. Amer. Chem. Soc. 102 (1980) 1563.
M. M. Reddy and G. H. Nancollas, J. Colloid Interface Sci. 36 (1971) 166.
R. G. Bates, “Determination of pH” (Wiley, New York, 1964).
G. H. Nancollas, Interactions in Electrolyte Solutions (Elsevier, Amsterdam, 1966).
H. S. Harned and S. R. Scholes, J. Amer. Chem. Soc. 63 (1941) 1706.
K. F. Wissbrun and D. M. French, J. Phys. Chem. 58 (1954) 693.
F. G. R. Gimblett and C. B. Monk, Trans. Farad. Soc. 50 (1954) 965.
R. M. Garrels and M. E. Thompson, Amer. J. Sci. 260 (1962) 57.
I. Greenwald, J. Biol. Chem. 141 (1941) 789.
H. S. Harned and W. J. Hamer, J. Amer. Chem. Soc. 55 (1933) 2194, 4496.
S. S. Zavodnov, Zhur. Vsesoyuz. Khin. Obshch. im D.I. Mendeleeva 9 (1964) 472.
R. F. Weiss, Marine Chem. 2 (1974) 203.
H. S. Harned and R. Davies, J. Amer. Chem. Soc. 65 (1943) 2030, 2037.
C. W. Davies, “Ion Association” (Butterworth, London, 1962).
G. H. Nancollas, Thalassia Jugoslavica 11 (12) (1975) 37.
P. G. Koutsoukos, Z. Amjad and G. H. Noncollas, J. Colloid Interface Sci., 83 (1981) 599.
S. M. Hamza, J. Cryst.Growth 102 (1990) 303.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Salem, M.R., Mangood, A.H. & Hamdona, S.K. Dissolution of calcite crystals in the presence of some metal ions. JOURNAL OF MATERIALS SCIENCE 29, 6463–6467 (1994). https://doi.org/10.1007/BF00354005
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00354005