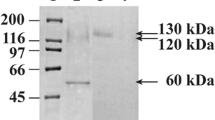
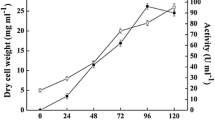
Abstract
A cDNA coding for glucoamylase P of Hormoconis resinae was cloned using a synthetic oligonucleotide probe coding for a peptide fragment of the purified enzyme and polyclonal anti-glucoamylase antibodies. Nucleotide-sequence analysis revealed an open reading frame of 1848 base pairs coding for a protein of 616 amino-acid residues. Comparison with other fungal glucoamylase amino-acid sequences showed homologies of 37–48%. The glucoamylase cDNA, when introduced into Saccharomyces cerevisiae under the control of the yeast ADC1 promoter, directed the secretion of active glucoamylase P into the growth medium.
Similar content being viewed by others
References
Ammerer G (1983) In: Wu R, Grossman L, Moldave K (eds) Methods Enzymol 101:192–201
Ashikari T, Nakamura N, Tanaka Y, Kiuchi N, Shibano Y, Tanaka T, Amachi T, Yoshizumi H (1985) Agric Biol Chem 49:2521–2523
Ashikara T, Nakamura N, Tanaka Y, Kiuchi N, Shibano Y, Tanaka T, Amachi T, Yoshizumi H (1986) Agric Biol Chem 50:957–964
Aviv H, Leder P (1972) Proc Natl Acad Sci USA 69:1408–1412
Ballance DJ (1986) Yeast 2:229–236
Beggs JD (1978) Nature 275:104–109
Birnboim HC, Doly J (1979) Nucleic Acids Res 7:1513–1523
Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ (1979) Biochemistry 18:5294–5299
Dente L, Cesareni G, Cortese R (1983) Nucleic Acids Res 11:1645–1655
Dohmen RJ, Strasser AWM, Dahlems UM, Hollenberg CP (1990) Gene 95:111–121
Fagerström R, Vainio A, Suoranta K, Pakula T, Kalkkinen N, Torkkeli H (1990) J Gen Microbiol 136:913–920
Gubler U, Hoffman BJ (1983) Gene 25:263–269
Gurr SJ, Unkles SE, Kinghorn JR (1987) The structure and organization of nuclear genes of filamentous fungi. In: Kinghorn JR (ed) Gene structure in eukaryotic microbes. IRL Press, Oxford, pp 93–139
Hamilton R, Watanabe CK, de Boer HA (1987) Nucleic Acids Res 15:3581–3593
Hanahan D (1983) J Mol Biol 166:557–580
Hashimoto H, Morikawa H, Yamada Y, Kimura A (1985) Appl Microbiol Biotechnol 21:336–339
Hejne G von (1983) Eur J Biochem 133:17–21
Innis MA, Holland MJ, McCabe PC, Cole GE, Wittman VP, Tal R, Watt KWK, Gelfand DH, Holland JP, Meade JH (1985) Science 228:21–26
Irniger S, Egli CM, Braus GH (1991) Mol Cell Biol 11:3060–3069
Itoh T, Ohtsuki I, Yamashita I, Fukui S (1987) J Bacteriol 169:4171–4176
Joutsjoki V, Torkkeli T (1992) FEMS Microbiol Lett 99:237–244
Kozak M (1981) Nucleic Acids Res 12:857–872
Laemmli UK (1970) Nature 227:680–685
Liljeström PL (1985) Nucleic Acids Res 13:7257–7268
Jiljeström-Suominen PL, Joutsjoki V, Korhola M (1988) Appl Env Microbiol 54:245–249
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Marshall JJ (1972) Wallerstein Labs Commun 35:49–98
McCleary BV, Anderson MA (1980) Carbohydr Res 86:77–96
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
de Mot R, van Dijk K, Donkers A, Verachtert H (1985) Appl Microbiol Biotechnol 22:222–226
Nunberg JH, Meade JH, Cole G, Lawyer FC, McCabe P, Schweickart V, Tal R, Wittman VP, Flatgaard JE, Innis MA (1984) Mol Cell Biol 4:2306–2315
Panchal CJ, Russell J, Sills AM, Stewart GG (1984) Food Technol 38:99–106
Sanger F, Nicklen S, Coulsen AR (1977) Proc Natl Acad Sci USA 74:5463–5467
Suominen PL (1988) Characterization and applications of the yeast MEL1 gene. PhD thesis, University of Helsinki
Svensson B, Sierks MR, Jespersen H, Søgaard M (1991) Structure-function relationships in amylases. In: Friedman RB (ed) Biotechnology of amylodextrin oligosaccharides. American chemical society, Washington, DC, pp 128–143
Tubb RS, Liljeström PL (1986) J Inst Brew 92:588–590
Yamashita I, Itoh T, Fukui S (1985a) Appl Microbiol Biotechnol 23:130–133
Yamashita I, Suzuki K, Fukui S (1985b) J Bacteriol 161:567–573
Yamashita I, Nakamura M, Fukui S (1987) J Bacteriol 169:2142–2149
Zagursky RJ, Berman ML, Baumeister K, Lomax N (1986) Gene Anal Tech 2:89–94
Zaret KS, Sherman F (1982) Cell 28:563–573
Author information
Authors and Affiliations
Additional information
Communicated by C. P. Hollenberg
Rights and permissions
About this article
Cite this article
Vainio, A.E.I., Torkkeli, H.T., Tuusa, T. et al. Cloning and expression of Hormoconis resinae glucoamylase P cDNA in Saccharomyces cerevisiae . Curr Genet 24, 38–44 (1993). https://doi.org/10.1007/BF00324663
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00324663