Summary
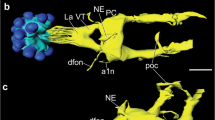
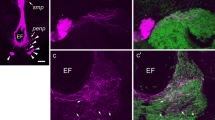
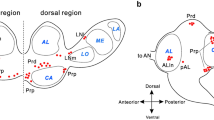
In a comparative study, the anatomy of neurons immunoreactive with an antiserum against the crustacean β-pigment-dispersing hormone was investigated in the brain of several orthopteroid insects including locusts, crickets, a cockroach, and a phasmid. In all species studied, three groups of neurons with somata in the optic lobes show pigment-dispersing hormone-like immunoreactivity. Additionally, in most species, the tritocerebrum exhibits weak immunoreactive staining originating from ascending fibers, tritocerebral cells, or neurons in the inferior protocerebrum. Two of the three cell groups in the optic lobe have somata at the dorsal and ventral posterior edge of the lamina. These neurons have dense ramifications in the lamina with processes extending into the first optic chiasma and into distal layers of the medulla. Pigment-dispersing hormone-immunoreactive neurons of the third group have somata near the anterior proximal margin of the medulla. These neurons were reconstructed in Schistocerca gregaria, Locusta migratoria, Teleogryllus commodus, Periplaneta americana, and Extatosoma tiaratum. The neurons have wide and divergent arborizations in the medulla, in the lamina, and in several regions of the midbrain, including the superior and inferior lateral protocerebrum and areas between the pedunculi and α-lobes of the mushroom bodies. Species-specific differences were found in this third cell group with regard to the number of immunoreactive cells, midbrain arborizations, and contralateral projections, which are especially prominent in the cockroach and virtually absent in crickets. The unusual branching patterns and the special neurochemical phenotype suggest a particular physiological role of these neurons. Their possible function as circadian pacemakers is discussed.
Similar content being viewed by others
References
Aréchiga H, Mena F (1975) Circadian variations of hormonal content in the nervous system of the crayfish. Comp Biochem Physiol 52A:581–584
Bonomelli SL, Rao KR, Riehm JP (1988) Development and application of an ELISA for crustacean β-PDH. Am Zool 28:117A
Campos-Ortega JA, Strausfeld NJ (1973) Synaptic connections of intrinsic cells and basket arborizations in the external plexiform layer of the fly's eye. Brain Res 59:119–136
Chiba Y, Tomioka K (1987) Insect circadian activity with special references to the localization of the pacemaker. Zool Sci 4:945–954
Colwell CS, Page TL (1990) A circadian rhythm in neural activity can be recorded from the central nervous system of the cockroach. J Comp Physiol [A] 166:643–649
Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP (1987) The ultrastructural of nerve endings containing pigment-dispersing hormone (PDH) in crustacean sinus glands: identification by an antiserum against synthetic PDH. Cell Tissue Res 250:377–387
Dores RM, Herman WS (1981) Insect chromatophorotropic factors: the isolation of polypeptides from Periplaneta americana and Apis mellifera with melanophore-dispersing activity in the crustacean Uca pugilator. Gen Comp Endocrinol 43:76–84
Ehnbohm K (1948) Studies on the central and sympathetic nervous system and some sense organs in the head of neuropteroid insects. Opusc Entomol Suppl 8:1–162
Fernlund P, Josefsson L (1972) Crustacean color change hormone: amino acid sequence and chemical synthesis. Science 177:173–175
Fingerman SW, Fingerman M (1977) Circadian variations in the levels of red pigment-dispersing hormone and 5-hydroxytryptamine in the eyestalks of the fiddler crab, Uca pugilator. Comp Biochem Physiol 56C:5–8
Fischbach K-F, Dittrich APM (1989) The optic lobe of Drosophila melanogaster. I. A. Golgi analysis of wild-type structure. Cell Tissue Res 258:441–475
Fleissner G (1982) Isolation of an insect circadian clock. J Comp Physiol [A] 149:311–316
Gäde G (1990) The adipokinetic hormone/red pigment concentrating hormone peptide family: structures, interrelationships, and functions. J Insect Physiol 36:1–12
Griss C, Rowell CHF (1986) Three descending interneurons reporting deviation from course in the locust. I. Anatomy. J Comp Physiol [A] 158:765–774
Hagberg M (1986) Ultrastructure and central projections of extraocular photoreceptors in caddisflies (Insecta: Trichoptera). Cell Tissue Res 245:643–648
Hanström B (1940) Inkretorische Organe, Sinnesorgane und Nervensystem des Kopfes einiger niederer Insektenordnungen. Kungl Sven Vetensk Akad Handl Ser 3, 18:1–265
Hedwig B (1986) On the role in stridulation of plurisegmental interneurons of the acridid grasshopper Omocestus viridulus. L. I. Anatomy and physiology of descending cephalothoracic interneurons. J Comp Physiol [A] 158:413–427
Hensler K (1988) The pars intercerebralis neurons PI(2)5 of locusts: convergent processing of inputs reporting head movements and deviations from straight flight. J Exp Biol 140:511–533
Hertel H, Maronde U (1987) Processing of visual information in the honeybee brain. In: Menzel R, Mercer A (eds) Neurobiology and behavior of honeybees. Springer, Berlin Heidelberg New York, pp 141–157
Hofbauer A, Buchner E (1989) Does Drosophila have seven eyes? Naturwissenschaften 76:335–336
Homberg U (1991) Neuroarchitecture of the central complex in the brain of the locust Schistocerca gregaria and S. americana as revealed by serotonin-immunocytochemistry. J Comp Neurol 303:245–254
Homberg U, Hildebrand JG (1989) Serotonin-immunoreactive neurons in the median protocerebrum and suboesophaeal ganglion of the sphinx moth, Manduca sexta. Cell Tissue Res 258:1–24
Järvilehto M (1985) The eye: vision and perception. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry, and pharmacology. Vol 5. Nervous system: structure and motor function. Pergamon, Oxford, pp 395–429
Klemm N, Steinbusch WM, Sundler F (1984) Distribution of serotonin-containing neurons and their pathways in the supraoesophageal ganglion of the cockroach Periplaneta americana (L.) as revealed by immunocytochemistry. J Comp Neurol 225:387–395
Koontz MA, Edwards JS (1980) The projection of neuroendocrine fibers (NCCI and II) in the brains of three orthopteroid insects. J Morphol 165:285–299
Larimer JL, Smith JTF (1980) Circadian rhythm of retinal sensitivity in crayfish: modulation by the cerebral and optic ganglia. J Comp Physiol 136:313–326
Loher W (1972) Circadian control of stridulation in the cricket Teleogryllus commodus Walker. J Comp Physiol 79:173–190
Mangerich S, Keller R, Dircksen H, Rao KR, Riehm JP (1987) Immunocytochemical localization of pigment-dispersing hormone (PDH) and its coexistence with FMRFamide-immunoreactive material in the eyestalks of the decapod crustaceans Carcinus maenas and Orconectes limosus. Cell Tissue Res 250:365–375
Meinertzhagen IA (1973) Development of the compound eye and optic lobe of insects. In: Young D (ed) Developmental neurobiology of arthropods. Cambridge University Press, Cambridge, pp 51–104
Mohrherr CJ, Rao KR (1985) Crustacean chromatophorotropic factors from the cricket, Acheta. Am Zool 25:83A
Nässel DR, Holmquist MH, Hardie RC, Håkanson R, Sundler F (1988) Histamin-like immunoreactivity in photoreceptors of the compound eyes and ocelli of the flies Calliphora erythrocephala and Musca domestica. Cell Tissue Res 253:639–646
Nowel MS, Shelton PMJ (1981) A Golgi-electron-microscopical study of the structure and development of the lamina ganglionaris of the locust optic lobe. Cell Tissue Res 216:377–401
Page TL (1978) Interactions between bilaterally paired components of the cockroach circadian system. J Comp Physiol 124:225–236
Page TL (1984) Neural organization of a circadian clock in the cockroach Leucophaea maderae. In: Porter R, Collins GM (eds) Photoperiodic regulation of insect and molluscan hormones. Pitman, London, pp 115–135
Page TL (1985) Clocks and circadian rhythms. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry, and pharmacology. Vol 6. Nervous system: sensory, Pergamon, Oxford, pp 577–652
Pflugfelder O (1936/37) Vergleichende, anatomische, experimentelle und embryologische Untersuchungen über das Nervensystem und ide Sinnesorgane der Rhynchoten. Zoologica 34:1–102
Pflugfelder O (1937) Die Entwicklung der optischen Ganglien von Culex pipiens. Zool Anz 117:31–36
Pipa L (1978) Locations and central projections of neurons associated with the retrocerebral neuroendocrine complex of the cockroach Periplaneta americana (L.). Cell Tissue Res 193:443–455
Rao KR (1985) Pigmentary factors. In: Bliss DE, Mantel LH (eds) The biology of Crustacea, vol 9. Academic Press, Orlando, pp 395–462
Rao KR, Riehm JP (1988a) Chemistry of crustacean chromatophorotropins. In: Bagnara JT (ed) Advances in pigment cell research. Liss, New York, pp 407–422
Rao KR, Riehm JP (1988 b) Pigment-dispersing hormones: a novel family of neuropeptides from arthropods. Peptides 9 [Suppl1]:153–159
Rao KR, Riehm JP (1989) The pigment-dispersing hormone family: chemistry, structure activity relations, and distribution. Biol Bull 177:225–229
Rao KR, Mohrherr CJ, Riehm JP, Zahnow CA, Norton S, Johnsson L, Tarr GE (1987) Primary structure of an analog of crustacean pigment-dispersing hormone from the lubber grasshopper Romalea microptera. J Biol Chem 262:2672–2675
Schulz W-D, Schlüter U, Seifert G (1984) Extraocular photoreceptors in the brain of Epilachna varivestis (Coleoptera, Coccinellidae). Cell Tissue Res 236:317–320
Sokolove PG (1975) Localization of the optic lobe circadian pacemaker with microlesions. Brain Res 87:13–21
Sternberger LA (1979) Immunocytochemistry. Wiley, New York
Strausfeld NJ (1976) Atlas of an insect brain. Springer, Berlin Heidelberg New York
Strausfeld NJ (1984) Functional neuroantomy of the blowfly's visul system. In: Ali MA (ed) Photoreception and vision in invertebrates. Plenum, New York, pp 483–522
Strausfeld NJ, Blest AD (1970) Golgi studies on insects. I. The optic lobes of Lepidoptera. Philos Trans R Soc Lond (Biol) 258:81–134
Thompson CS, Lococo DJ, Tobe SS (1987) Anatomy and electrophysiology of neurons terminating in the corpora allata of the cockroach Diploptera punctata. J Comp Neurol 261:120–129
Wiedenmann G (1980) Two peaks in the activity rhythm of cockroaches controlled by one circadian pacemaker. J Comp Physiol 137:249–254
Wiedenmann G (1983) Splitting in a circadian activity rhythm: the expression of bilaterally paired oscillators. J Comp Physiol [A] 150:51–60
Williams JLD (1975) Anatomical studies of the insect central nervous system: a ground-plan of the midbrain and an introduction to the central complex in the locust, Schistocerca gregaria (Orthoptera). J Zool Lond 76:67–86
Wills SA, Page TL, Colwell CS (1985) Circadian rhythms in the electroretinogram of the cockroach. J Biol Rhythms 1:25–37
Würden S, Homberg U, Dircksen H, Rao KR (1990) Comparative morphology of PDH-immunoreactive neurons in the brain of orthopteroid insects. In: Elsner N, Roth G (eds) Brain-perception-cognition. Thieme, Stuttgart, p 316
Zahnow CA, Rao KR, Mohrherr CJ, Riehm JP (1987) Immunocytochemistry of neuropeptides in the cephalic neuroendocrine system of the grasshopper, Romalea microptera. Soc Neurosci Abstr 13:993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Homberg, U., Würden, S., Dircksen, H. et al. Comparative anatomy of pigment-dispersing hormone-immunoreactive neurons in the brain of orthopteroid insects. Cell Tissue Res 266, 343–357 (1991). https://doi.org/10.1007/BF00318190
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318190