Abstract
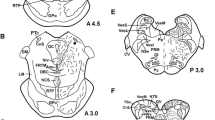
The distribution of noradrenaline and adrenaline in the brain of the urodele amphibian Pleurodeles waltlii has been studied with antibodies raised against noradrenaline and the enzymes dopamine-β-hydroxylase and phenylethanolamine-N-methyltransferase. Noradrenaline-containing cell bodies were found in the anterior preoptic area, the hypothalamic nucleus of the periventricular organ, the locus coeruleus and in the solitary tract/area postrema complex at the level of the obex. Noradrenergic fibers are widely distributed throughout the brain innervating particularly the ventrolateral forebrain, the medial amygdala, the lateral part of the posterior tubercle, the parabrachial region and the ventrolateral rhombencephalic tegmentum. Putative adrenergic cell bodies were found immediately rostral to the obex, ventral to the solitary tract. Whereas the cell bodies and their dendrites were Golgi-like stained, axons were more difficult to trace. Nevertheless, some weakly immunoreactive fibers could be traced to the basal forebrain. A comparison of these results with data previously obtained in anurans reveals not only several general features, but also some remarkable species differences.
Similar content being viewed by others
Abbreviations
- Acc :
-
Nucleus accumbens
- AP :
-
area postrema
- Apl :
-
amygdala, pars lateralis
- Apm :
-
amygdala, pars medialis
- ca :
-
commissura anterior
- Cb :
-
cerebellum
- cc :
-
central canal
- Dp :
-
dorsal pallium
- epl :
-
external plexiform layer
- gl :
-
glomerular layer of the olfactory bulb
- H :
-
ganglion habenulae
- igl :
-
internal granular layer
- Ip :
-
nucleus interpeduncularis
- Lc :
-
locus coeruleus
- Ll :
-
lateral line lobe
- Lp :
-
lateral pallium
- Ls :
-
lateral septum
- ml :
-
mitral cell layer
- Mp :
-
medial pallium
- Ms :
-
medial septum
- nPT :
-
nucleus pretectalis
- NPv :
-
nucleus of the periventricular organ
- nV :
-
nervus trigeminus
- oc :
-
optic chiasm
- Poa :
-
preoptic area
- Ri :
-
nucleus reticularis inferior
- SC :
-
nucleus suprachiasmaticus
- sol :
-
solitary tract
- Str :
-
striatum
- thd:
-
thalamus dorsalis
- thv :
-
thalamus ventralis
- To :
-
tectum opticum
- TP :
-
tuberculum posterius
- V :
-
ventricle
- VH :
-
ventral hypothalamic nucleus
- III :
-
nucleus nervi oculomotorii
- IXm :
-
nucleus motorius nervi glossopharyngei
- Xm :
-
nucleus motorius nervi vagi
References
Berger B, Gaspar, P (1994) Comparative anatomy of the catecholaminergic innervation of rat and primate cerebral cortex. In: Smeets WJAJ, Reiner A (eds) Phylogeny and development of catecholamine systems in the CNS of vertebrates. Cambridge University Press, Cambridge, pp 293–324
Cooney MM, Conaway CH, Mefford IN (1985) Epinephrine, norepineprhine and dopamine concentrations in amphibian brain. Comp Biochem Physiol [C] 82:395–397
Corio M, Thibault J, Peute J (1990) Topographical relationships between catecholamine- and neuropeptide-containing fibers in the median eminence of the newt, Triturus alpestris. An ultrastructural immunocytochemical study. Cell Tissue Res 259:561–566
Corio M, Thibault J, Peute J (1992) Distribution of catecholaminergic and serotoninergic systems in forebrain and midbrain of the newt, Triturus alpestris (Urodela). Cell Tissue Res 268:377–387
Franzoni MF, Thibault J, Fasolo A, Martinoli MG, Scanari F, Calas A (1986) Organization of tyrosine-hydroxylase immunopositive neurons in the brain of the crested newt, Triturus cristatus carnifex. J Comp Neurol 251:121–134
González A, Smeets WJAJ (1991) Comparative analysis of dopamine and tyrosine hydroxylase immunoreactivities in the brain of two amphibians, the anuran Rana ridibunda and the urodele Pleurodeles waltlii. J Comp Neurol 303:457–477
González A, Smeets WJAJ (1993) Noradrenaline in the brain of the South African clawed frog Xenopus laevis. A study with antibodies against noradrenaline and dopamine-β-hydroxylase. J Comp Neurol 331:363–374
González A, Tuinhof R, Smeets WJAJ (1993) Distribution of tyrosine hydroxylase- and dopamine-immunoreactivities in the brain of the South African clawed frog Xenopus laevis. Anat Embryol 187:193–201
Meek J, Joosten HWJ, Hafmans TGM (1993) Distribution of noradrenaline-immunoreactivity in the brain of the mormyrid teleost Gnathonemus petersii. J Comp Neurol 328:145–160
Smeets WJAJ (1994) Catecholamine systems in the CNS of reptiles. In: Smeets WJAJ, Reiner A (eds) Phylogeny and development of catecholamine systems in the CNS of vertebrates. Cambridge University Press, Cambridge, pp 103–133
Smeets WJAJ, González A (1990) Are putative dopamine-accumulating cell bodies in the hypothalamic periventricular organ a primitive brain character of nonmammalian vertebrates? Neurosci Letter 114:248–252
Smeets WJAJ, Jonker A (1990) Distribution of phenylethanolamine-N-methyltransferase-immunoreactive perikarya and fibers in the brain of the lizard Gekko gecko. Brain Behav Evol 36:59–72
Smeets WJAJ, Steinbusch HWM (1989) Distribution of noradrenaline immunoreactivity in the forebrain and midbrain of the lizard Gekko gecko. J Comp Neurol 285:453–466
Smeets WJAJ, Steinbusch HWM (1990) New insights into the reptilian catecholaminergic systems as revealed by antibodies against the neurotransmitters and their synthetic enzymes. J Chem Neuroanat 3:25–43
Steinbusch HWM, FJ Tilders (1987) Immunohistochemical techniques for light-microscopical localization of dopamine, noradrenaline, adrenaline, serotonin and histamine in the central nervous system. In: Steinbusch HWM (ed) Methods in neurosciences, vol 10, Monoaminergic neurons: light microscopy and ultrastructure. Wiley, Chichester, pp 125–166
Vehofstad AAJ, Steinbusch HWM, Penke B, Varga J, Joosten HWJ (1980) Use of antibodies to norepinephrine and epinephrine in immunohistochemistry. In: Eränkö O, Soinila S, Paivarinta H (eds) Histochemistry and cell biology of autonomic neurons, SIF cells, and paraneurons. Raven Press, New York, pp 185–193
Verhofstad AAJ, Steinbusch HWM, Joosten HWJ, Penke B, Varga J, Goldstein M (1982) Immunocytochemical localization of noradrenaline, adrenaline, and serotonin. In: Polak JM, van Noorden S (eds) Immunocytochemistry: practical applications in pathology and biology. Wright, Bristol, pp 143–168
Yoshida M, Nagatsu I, Kondo Y, Karasawa N, Ohno T, Spatz M, Nagatsu T (1983) Immunohistochemical localization of the neurons containing catecholamine-synthesizing enzymes and serotonin in the brain of the bullfrog (Rana catesbeiana). Acta Histochem Cytochem 16:245–258
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
González, A., Smeets, W.J.A.J. Noradrenergic and adrenergic systems in the brain of the urodele amphibian, Pleurodeles waltlii, as revealed by immunohistochemical methods. Cell Tissue Res 279, 619–627 (1995). https://doi.org/10.1007/BF00318174
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318174