Abstract
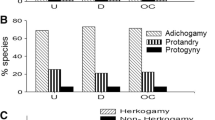
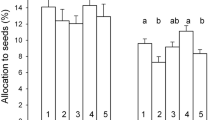
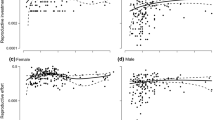
Components of the total sexual investment of plants growing under mediterranean climatic and edaphic conditions were compared with those of plants growing in the desert, in the closely related allogamous species pair Erucaria hispanica and E. rostrata and in populations of the autogamous species Erophila minima. In all cases lower total investment was evident in the desert plants. At the prezygotic phase it was expressed by (1) reduced production of flowers; (2) lower allocation to the production of male gametophytes and some floral organs; and (3) packaging of more ovules per ovary. The ratio of reproductive: vegetative biomass which was found to be greater in the desert plants and their lower pollen: ovule ratio are perhaps indicative of greater efficiency. Their smaller investment at the postzygotic phase was expressed in: (1) reduction in total numbers of fruits and seeds; (2) decrease in seed size and weight. Yet in the desert plants the number of seeds per total biomass was found to be significantly larger and fertility rates (seed-set per ovule, fruit-set per flower per plant) were equal to or greater than those in the mediterranean plants. The trends observed in this study in desert plants, which may result in more efficient exploitation of resources, are similar in the species involved, regardless of their breeding system-autogamous or allogamous.
Similar content being viewed by others
References
Ackerman JD, Montalvo AM (1990) Short- and long-term limitations to fruit production in a tropical orchid. Ecology 71: 263–272
Armstrong-Goldman DW, Willson MF, (1986) Sex allocation in functionally hermaphroditic plants: a review and critique. Bot Rev 52: 157–194
Bawa KS, Beach JH (1981) Evolution of sexual systems in flowering plants. Ann Mo Bot Gard 68: 254–274
Bertin RI (1988) Paternity in plants. In: Lovett-Doust J, Lovett-Doust L (eds) Plant reproductive ecology. Oxford University Press, Oxford, pp 30–59
Boaz M (1989) Sexual reproductive effort and its allocations in Cruciferae plants in different phytogeographical territories. PhD thesis, The Hebrew University, Jerusalem
Boaz M, Plitmann U, Heyn CC (1990) The ecogeographic distribution of breeding systems in the Cruciferae (Brassicaceae) of Israel. Isr J Bot 39: 31–42
Bradshaw AD (1965) Evolutionary significance of phenotypic plasticity in plants. Adv Genet 13: 115–155
Campbell DR (1992) Variation in sex allocation and floral morphology in Ipomopsis aggregata (Polemoniaceae). Am J Bot 79: 516–521
Campbell DR, Halama KJ (1993) Resource and pollen limitations to lifetime seed production in natural plant population. Ecology 74: 1043–1051
Charlesworth D, Charlesworth B (1987) The effect of investment in attractive structures on allocation to male and female functions in plants. Evolution 41: 948–968
Charnov EL (1982) The theory of sex allocation (Monographs in Population Biology 18). Princeton University Press, Princeton
Charnov EL (1987) On sex allocations and selfing in higher plants. Evol Ecol 1: 30–36
Cheplick GP, Quinn JA (1982) Amphicarpum purshii and the “pessimistic strategy” in amphicarpic annuals with subterranean fruits. Oecologia 52: 327–332
Cohen D (1971) Maximizing final yields when growth is limited by time or by limiting resources. J Theor Biol 33: 299–307
Cruden RW (1977) Pollen-ovule ratio: a conservative indicator of breeding systems in flowering plants. Evolution 31: 32–46
Cruden RW, Lyons DL (1985) Patterns of biomass allocation to male and female functions in plants with different mating systems. Oecologia 66: 299–306
Cumaraswamy A, Bawa KS (1989) Sex allocation and mating systems in pigeonpea, Cajanus cajan (Fabaceae). Plant Syst Evol 168: 59–69
Dale MFP, Ford-Lloyd BV (1983) Reproductive characters associated with breeding behavior in Beta sect. Beta (Chenopodiaceae). Plant Syst Evol 143: 277–283
Devlin B, Ellstrand N (1990). Male and female fertility variation in wild radish, a hermaphrodite. Am Nat 136: 87–107
Dunn CP, Sharitz RR (1991) Population structure, biomass allocation and phenotypic plasticity in Murdannia keisak (Commelinaceae). Am J Bot 78: 1712–1723
Evenson WE (1983) Experimental studies of reproductive energy allocation in plants. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand-Reinhold, New York, pp 249–274
Galen C, Newport MEA (1988) Pollination quality, seed set and flower traits in Polemonium viscosum: complementary effects of variation in flower scent and size. Am J Bot 75: 900–905
Hedge IC, Lamond JM (1980) Cruciferae — Brassiceae. In: Townsend CC, Guest E (eds) Flora of Iraq, vol 4. Ministry of Agriculture, Baghdad, pp 827–885
Hickman JC (1975) Environmental unpredictability and plastic energy allocation strategies in the annual Polygonum cascadense (Polygonaceae). J Ecol 63: 689–701
Hickman JC, Pitelka LF (1975) Dry weight indicates energy allocation in ecological analysis of plants. Oecologia 21: 117–121
Jain SK (1976) The evolution of inbreeding in plants. Annu Rev Ecol Syst 74: 469–495
Kang H, Primack RB (1991) Temporal variation of flower and fruit size in relation to seed yield in celandine poppy (Chelidonium majus, Papaveraceae). Am J Bot 78: 711–722
Kay QON (1987) The comparative ecology of flowering. New Phytol 106: 265–281
Kozlowski J, Wieger RG (1986) Optimal allocation of energy to growth and reproduction. Theor Popul Biol 29: 16–37
Lloyd DG (1979) Parental strategies in Angiosperms. NZ J Bot 17: 595–606
Lloyd DG (1980) Demographic factors and mating patterns in Angiosperms. Bot Monog 15: 67–88
Lloyd DG (1984) Variation strategies of plants in heterogeneous environments. Biol J Linn Soc 21: 357–385
Lovett-Doust J, Cavers PB (1982) Biomass allocation to hermaphrodite flowers. Can J Bot 60: 2530–2534
Lyons EE, Antonovics J (1991) Breeding system evolution in Leavenworthia: Breeding system variation and reproductive success in natural populations of Leavenworthia crassa (Cruciferae). Am J Bot 78: 270–287
Mione T, Anderson GJ (1992) Pollen: ovule ratios and breeding system evolution in Solanum section Basarthrum (Solanaceae). Am J Bot 79: 279–298
Morita T, Hiratsuka A, Masuzawa T, Kawano S (1993) Chasmogamy vs. cleistogamy — the adaptive significance of dimorphic reproduction, with special reference to Leibnitzia anandria, a Compositae perennial (abstract). Proc. XV International Bot Congr, Yokohama, Japan, p 36
Newel SJ, Tramer EJ (1978) Reproductive strategies in herbaceous plants communities during succession. Ecology 59: 228–234
Noy-Meir I (1986) Desert ecosystems: structure and function. In: Evenari M, Noy-Meir I, Goodall DW (eds) Hot deserts and arid shrublands. Elsevier, Amsterdam, pp 93–103
Pitelka LF (1977) Energy allocation in annual and perennial lupines (Lupinus, Leguminosae). Ecology 58: 1055–1065
Queller DC (1983) Sexual selection in a hermaphroditic plant. Nature 305: 706–707
Richards (1986) Plant breeding systems. Allen and Unwin, London
Samson DA, Werk KS (1986) Size dependent effects in the analysis of reproductive effort in plants. Am Nat 172: 667–680
Schlichting CD (1986) The evolution of phenotypic plasticity in plants. Annu Rev Ecol Syst 17: 667–693
Schoen DJ (1982) Male reproductive effort and breeding system in a hermaphroditic plant. Oecologia 53: 255–257
Shmida A, Evenari M, Noy-Meir I (1986) Hot desert ecosystem: an integrated view. In: Evenari M, Noy-Meir I, Goodall DW (eds) Hot deserts and arid shrublands. Elsevier, Amsterdam, pp 379–387
Sokal RR, Rohlf FJ (1982) Biometry, Freeman, San Francisco
Solbrig OT (1976) On the relative advantages of cross and self fertilization. Ann Mo Bot Gard 63: 262–276
Stanton ML, Galloway LF (1990) Natural selection and the allocation to reproduction in flowering plants. In: Mangel M (ed) Sex allocation and sex change experiments and models. American Mathematical Society, Providence, pp 1–50
Stanton ML, Snow AA, Handel SN (1986) Floral evolution: attractiveness to pollinators increases male fitness. Science 232: 1625–1627
Taylor DR, Aarsen LW (1988) An interpretation of phenotypic plasticity in Agropyron repens (Gramineae). Am J Bot 66: 313–320
Waller DM (1979) The relative costs of self and cross fertilized seeds in Impatiens capensis (Balsaminaceae). Am J Bot 66: 313–320
Waller DM (1988) Plant morphology and reproduction. In: Lovett-Doust J, Lovett-Doust L (eds) Plant reproductive ecology. Oxford University Press, pp 203–227
Waser NM, Price MV (1991) Reproductive costs of self pollination in Ipomopsis aggregata (Polemoniaceae). Are ovules usurped? Am J Bot 78: 1036–1043
Wiens D (1984) Ovule survivorship, brood size, life history, breeding systems and reproductive success in plants. Oecologia 64: 47–53
Willson MF (1983) Plant reproductive ecology. Wiley, New York
Wyatt R (1982) Inflorescence architecture: how flower number, arrrangement and phenology affect pollination and fruit-set. Am J Bot 69: 585–594
Wyatt R (1984) Evolution of self-pollination in granite outcrop species of Arenaria (Caryophyllaceae). III. Reproductive effort and pollen-ovule ratios. Syst Bot 9: 432–440
Zar JH (1984) Biostatical analysis. Prentice Hall, Englewood
Zohary M (1966) Flora Palaestina, vol. 1. Israel Academy of Sciences, Jerusalem
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boaz, M., Plitmann, U. & Heyn, C.C. Reproductive effort in desert versus mediterranean crucifers: the allogamous Erucaria rostrata and E. hispanica and the autogamous Erophila minima . Oecologia 100, 286–292 (1994). https://doi.org/10.1007/BF00316956
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00316956