Summary
The pharmacokinetics and β-adrenoceptor blocking effects of conventional and sustained-release metipranolol have been studied in 6 healthy male volunteers given a single oral dose of 40 mg. Plasma drug concentrations determined by TLC and a radioreceptor assay, and the inhibition of exercise-induced tachycardia, were monitored for 48 h.
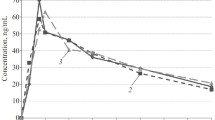
Relevant amounts of active metabolites other than deacetylmetipranolol were not found. Compared to conventionally formulated metipranolol, the controlled-release product had a prolonged mean residence time (10.7 vs 5.5 h), the peak drug concentration was halved and the time to peak drug concentrations was delayed. Relatively constant plasma concentrations (cideal = 6.5 ng/ml) and a clinically significant reduction of exercise-induced tachycardia were maintained throughout a 24 h dosing interval. An individual deacetylmetipranolol plasma concentration-effect relationship was evaluated using the Emax model. Mean parameters were Emax 26% and C50 2.9 ng/ml.
Similar content being viewed by others
References
Aellig WH, Narjes HH, Nüesch E, Oertle RJ, Devos JE, Pacha W (1981) A pharmacodynamic and pharmacokinetic comparison of pindolol 20 mg retard and conventional tablet. Eur J Clin Pharmacol 20: 179–183
Boxenbaum H (1984) Pharmacokinetic determinants in the design and evaluation of sustained-release dosage forms. Pharm Res 2: 82–88
Brockmeier D, Hajdu P, Henke W, Mutschler E, Palm D, Rupp W, Spahn H, Verho MT, Wellstein A (1988) Penbutolol: Pharmacokinetics, effect on exercise tachycardia, and in vitro inhibition of radioligand binding. Eur J Clin Pharmacol 35: 613–623
Cheymol G, Jaillon P, Lecoq B, Cheymol A, Krumenacker M (1987) Cardiovascular β-adrenergic blocking effects of bornaprolol in humans: Relation to dose and plasma concentration. J Cardiovasc Pharmacol 9: 694–698
Chidsey CA, Morselli P, Bianchetti G, Morganti A, Leonetti G, Zanchetti A (1975) Studies on the absorption and disposition of propranolol in hypertensive patients during therapy. Circulation 52: 313–318
Garg DC, Jallad NS, Mishriki A, Chalasarya G, Kraml M, Fencik M, Weidler DJ (1987) Comparative pharmacodynamics and pharmacokinetics of conventional and long-acting propranolol. J Clin Pharmacol 27: 390–396
Gibaldi M, Perrier D (1975) Pharmacokinetics. Dekker, New York
Hager WD, Pieniascek HJ, Perrier D, Mayersohn M, Goldberger V (1981) Assessment of beta blockade with propranolol. Clin Pharmacol Ther 30: 283–290
Holford NH, Sheiner LB (1982) Kinetics of pharmacologic response. Pharmacol Ther 16: 143–166
Lalonde RL, Pieper JA, Straka RJ, Bottorff MB, Mirvis DM (1987 a) Propranolol pharmacokinetics and pharmacodynamics after single doses and at steady-state. Eur J Clin Pharmacol 32: 315–318
Lalonde RL, Straka RJ, Pieper JA, Bottorff MB, Mirvis DM (1987 b) Propranolol pharmacodynamic modeling using unbound and total concentrations in healthy volunteers. J Pharmacokinet Biopharm 15: 569–582
Marino MR, Dey M, Garg DC, Jallad NS, Dorick DM, Martinez JJ, Weidler DJ (1987) Pharmacokinetics and pharmacodynamics of long-acting propranolol 60-mg capsules: A comparative evaluation. J Clin Pharmacol 27: 885–891
Mayer O, Čepelák V, Vitouš J, Potměšil J (1980) β-blocking drug metipranolol: Plasma levels and pharmacodynamic action in man. Int J Clin Pharmacol Ther Toxicol 18: 113–120
Meier J, Nüesch E, Schmidt R (1974) Pharmacokinetic criteria for the evaluation of retard formulations. Eur J Clin Pharmacol 7: 429–432
Nace GS, Wood AJJ (1987) Pharmacokinetics of long acting propranolol: Implications for therapeutic use. Clin Pharmacokinet 13: 51–64
Nimmerfall F, Rosenthaler J (1986) Modified release of drugs: a way to its quantification. Int J Pharm 32: 1–6
Peterková M, Matoušová O, Rejholec V (1986) Densitometric determination of deacetylmetipranolol in blood plasma (In Czech). Ceskoslov Farm 35: 456–457
Platzer R, Galeazzi RL, Niederberger W, Rosenthaler J (1984) Simultaneous modeling of bopindolol kinetics and dynamics. Clin Pharmacol Ther 36: 5–13
Riegelman S, Collier P (1980) The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokinet Biopharm 8: 509–534
Španěl M, Čepeláková H (1989) The determination of pharmacokinetic paramaters of Trimepranol and Trimepranol retard by a radioreceptor assay (In Czech). Českoslov Fysiol 38: 175
Vallner JJ, Honigberg IL, Kotzan JA, Stewart JT (1983) A proposed general protocol for testing bioequivalence of controlled-release drug products. Int J Pharm 16: 47–55
Wellstein A, Palm D, Pitschner HF, Belz GG (1985) Receptor binding of propranolol is the missing link between plasma concentration kinetics and the effect-time course in man. Eur J Clin Pharmacol 29: 131–147
Wellstein A, Palm D, Wiemer G, Schäfer-Korting M, Mutschler E (1984) Simple and reliable radioreceptor assay for β-adrenoreceptor antagonists and active metabolites in native human plasma. Eur J Clin Pharmacol 27: 545–553
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lapka, R., Sechser, T., Rejholec, V. et al. Pharmacokinetics and pharmacodynamics of conventional and controlled-release formulations of metipranolol in man. Eur J Clin Pharmacol 38, 243–247 (1990). https://doi.org/10.1007/BF00315024
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315024