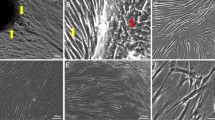
Summary
Numerous metabolic studies have demonstrated heterogeneity of fibroblast populations in culture, yet little is known about the structure of fibroblast populations in adult tissues in vivo. To determine if populations of both cycling and non-cycling cells are present in gingiva, hamsters were labelled with [3H]-thymidine to label cycling cells in vivo, and explanted biopsies were subsequently incubated with bromodeoxyuridine to label cycling cells in vitro. Cycling cells were identified by combined immunohistochemistry and radioautography. Fibroblasts were recognized by the presence of vimentin and the absence of keratin as determined by immuno-fluorescence. The largest proportion of cells were double-labelled with [3H]-thymidine and bromodeoxyuridine (43.8%) indicating the presence of actively cycling populations that maintained their proliferative status upon explantation. Cultures also exhibited a second population of cells labelled only with bromodeoxyuridine (38.7%) that did not cycle in vivo, but retained the capacity for proliferation in vitro. However, limiting dilution analysis of single-cell suspensions revealed only a single class of progenitors capable of forming large colonies in vitro. Approximately 1 in 190 plated cells was capable of colony-formation, indicating that, upon explantation, a subset of the cycling cells in vitro exhibits extensive proliferative capacity. There was also a small population of cells unlabeled with either [3H]-thymidine or bromodeoxyuridine (9.4%) that appeared to be terminally differentiated. Different substrates, including glass and thin films of gelatin and collagen, did not significantly alter the fraction of cells labelled with [3H]-thymidine. These data demonstrate the existence of 2 separate progenitor-cell populations with different capacities for proliferation in vivo and in vitro.
Similar content being viewed by others
References
Barendsen GW, Roelse H, Hermens AF, Madhuizen HT, van Peperzeel HA, Rutgers DH (1973) Clonogenic capacity of proliferating and nonproliferating cells of a transplantable rat rhabdomyosarcoma in relation to its radiosensitivity. J Natl Cancer Inst 51:1521–1526
Bayreuther K, Rodemann P, Francz PI, Maier K (1988a) Differentiation of fibroblast stem cells. J Cell Sci Suppl 10:115–130
Bayreuther K, Rodemann P, Hommel R, Dittmann K, Albeiz M, Francz PI (1988b) Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci USA 85:5112–5116
Bohmer RM, Beattie LD (1988) Probability of transition into cell cycle does not depend on growth factor concentration. J Cell Physiol 136:194–197
Bordin S, Page RC, Narayanan AS (1984) Heterogeneity of normal human diploid fibroblasts: isolation and characterization of one phenotype. Science 223:171–173
Burmer G, Norwood TH (1980) Selective elimination of proliferating cells in human diploid cell cultures by treatment with BrdU, 33258 Hoechst and visible light. Mech Ageing Dev 12:151–159
Chen TR (1977) In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res 104:255–262
Cleaver JF (1967) Thymidine metabolism and cell kinetics. North Holland, Amsterdam
Connor NS, Aubin JE, Sodek J (1983) Independent expression of type I collagen and fibronectin by normal fibroblast-like cells. J Cell Sci 63:233–244
Ehmann UK, Williams JR, Nagle WA, Brown JA, Belli JA, Lett JT (1975) Perturbations in cell cycle progression from radioactive precursors. Nature 258:633–636
Elmore E, Swift M (1977) Growth of human skin fibroblasts in dialyzed fetal bovine serum. In Vitro 13:837–842
Friedenstein AJ, Chailakhjan RL, Lalykina KS (1970) The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3:393–403
Harper RA, Grove G (1979) Human skin fibroblasts derived from papillary and reticular dermis: differences in growth potential in vitro. Science 204:526–527
Hassell TM, Stanek III EJ (1983) Evidence that healthy human gingiva contains functionally heterogenous fibroblast subpopulations. Arch Oral Biol 28:617–625
Hoerl B, Scott RE (1989) Nonterminally differentiated cells express decreased growth factor responsiveness. J Cell Physiol 139:68–75
Holliday R, Huschtscha LI, Kirkwood TBL (1981) Cellular aging: further evidence for the commitment theory. Science 213:1505–1508
Hume WJ (1989) DNA-synthesizing cells in oral epithelium have a range of cell cycle durations: evidence from double-labelling studies using tritiated thymidine and bromodeoxyuridine. Cell Tissue Kinet 22:377–382
Hume WJ, Saffhill R (1986) Iodo- and bromodeoxyuridine are excised at different rates from DNA of mouse tongue keratinocytes in vitro. Chem Biol Interact 60:227–232
Hume WJ, Thompson J (1989) Double labelling of tissue combining tritated thymidine autoradiography with immunodetection of bromodeoxyuridine: the autoradiographic significance of inhibition of thymidine incorporation into DNA by bromode-oxyuridine given simultaneously. Cell Tissue Kinet 22:393–399
Kleinman HK, Luckenbill-Edds L, Cannon FW, Sephel GC (1987) Use of extracellular matrix components for cell culture. Anal Biochem 166:1–13
Korn JH, Downie E (1989) Clonal interactions in fibroblast proliferation: recognition of self vs. non-self. J Cell Physiol 141:437–440
Korn JH, Halushka PV, Le Roy EC (1980) Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogeneous prostaglandin production. J Clin Invest 65:543–554
Korn JH, Torres D, Downie E (1984) Clonal heterogeneity in the fibroblast response to mononuclear cell derived mediators. Arthritis Rheum 27:174–179
Lajtha LG (1979) Stem cell concepts. Differentiation 14:23–34
Lefkovits I, Waldmann H (1979) Limiting dilution analysis of cells in the immune system. University Press, London Cambridge, pp 38–81
Limeback H, Sodek J, Aubin JE (1983) Variation in collagen expression by cloned periodontal ligament cells. J Periodontol Res 18:242–248
Martin GM, Spraque CA, Norwood TH, Pendergrass WR (1974) Clonal selection, attenuation and differentiation as an in vitro model for hyperplasia. Am J Pathol 74:37–154
McCulloch CAG (1986) Effect of experimental periodontitis on fibroblast progenitor populations in hamster gingiva. J Periodontol Res 21:685–691
McCulloch CAG, Melcher AH (1983a) Cell density and cell generation in the periodontal ligament of mice. Am J Anat 167:43–58
McCulloch CAG, Melcher AH (1983b) Continuous labelling of the periodontal ligament of mice. J Periodontol Res 18:231–241
McCulloch CAG, Knowles G, Overall CM (1987) Quantitation and optimization of enzymatic and mechanical procedures to produce high-yield single cell suspensions from human gingiva. J Periodontol Res 22:41–49
Narayanan AS, Page RC (1983) Connective tissues of the periodontium: a summary of current work. Collagen Rel Res 3:33–64
Rabinovitch PS (1983) Regulation of human fibroblast growth rate by both noncycling cell fraction and transition probability is shown by growth in 5-bromodeoxyuridine followed by Hoechst 33258 flow cytometry. Proc Natl Acad Sci USA 80:2951–2955
Rhudy RW, McPherson JM (1988) Influence of the extracellular matrix on the proliferative response of human skin fibroblasts to serum and purified platelet-derived growth factor. J Cell Physiol 137:185–191
Roberts WE (1975) Cell kinetic nature and diurnal periodicity of rat periodontal ligament. Archs Oral Biol 20:465–471
Rubin H, Nomura T (1987) Use of lymph in cell culture to model hormonal and nutritional constraints on tumor growth in vivo. Cancer Res 47:4924–4931
Russell JD, Russell SB, Tropin KM (1982) Fibroblast heterogeneity in glucocorticoid regulation of collagen metabolism: genetic or epigenetic. In Vitro 18:557–564
Safhill R, Ockey CH (1985) Strand breaks arising from the repair of the 5-bromodeoxyuridine-substituted template and methyl methanesulphonate-induced lesions can explain the formation of sister chromatid exchanges. Chromosoma 92:218–224
Schor SL (1980) Cell proliferation and migration on collagen substrata in vitro. J Cell Sci 41:159–175
Schor SL, Schor AM (1987) Clonal heterogeneity in fibroblast phenotype: implications for the control of epithelial-mesenchymal interactions. Bioessays 7:200–204
Smith JR, Hayflick L (1984) Variation in the lifespan of clones derived from human diploid strains. J Cell Biol 62:48–53
Umeno Y, Okuda A, Kimura G (1989) Proliferative behaviour of fibroblasts in plasma-rich culture medium. J Cell Sci 94:567–575
Zavala C, Herner G, Fialkow PJ (1978) Evidence for selection in cultured diploid fibroblast strains. Exp Cell Res 117:137–144
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McCulloch, C.A.G., Knowles, G. Discrimination of two fibroblast progenitor populations in early explant cultures of hamster gingiva. Cell Tissue Res 264, 87–94 (1991). https://doi.org/10.1007/BF00305725
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00305725