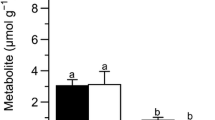
Abstract
The effects of anoxia (N2 atmosphere at 5 °C) or freezing (at-8 °C) exposure in vivo on the activities of five enzymes of carbohydrate metabolism were assessed in foot muscle and hepatopancreases of the marine periwinkle Littorina littorea. Changes in glycogen phosphorylase, glycogen synthetase, pyruvate kinase and pyruvate dehydrogenase under either stress were generally consistent with covalent modification of the enzymes to decrease enzyme activity and/or convert the enzyme to a less active form. However, no evidence for a similar covalent modification of phosphofructokinase was found. The metabolic effects of freezing and anoxia were generally similar, suggesting that a primary contributor to freezing survival is the implementation of anaerobic metabolism and metabolic arrest mechanisms that also promote anoxia survival in marine molluses. However, in hepatopancreas phosphorylase was activated and pyruvate kinase remained in two enzyme forms in freezing-exposed snails, contrary to the results for anoxic animals. Ion exchange chromatography on DE-52 Sephadex revealed the presence of two forms of pyruvate kinase in both tissues of control L. littorea, eluting at 30–50 mmol·1-1 KCl (peak I) or 90–110 mmol·1-1 KCl (peak II). Anoxia exposure converted pyruvate kinase in both tissues to the peak I form, as did freezing for foot muscle pyruvate kinase. Kinetic analysis showed that peak I pyruvate kinase had lower affinities for substrates, phosphoenolpyruvate and ADP, and was very strongly inhibited by l-alanine compared with the peak II enzyme. Peak I pyruvate kinase had an I 50 value for l-alanine of 0.38 mmol·1-1, whereas peak II pyruvate kinase was unaffected by l-alanine evenat 40 mmol·1-1. In vitro incubation of extracts from control foot muscle under conditions promoting phosphorylation or dephosphorylation identified the peak I and II forms as the low and high phosphate forms, respectively. This result for L. littorea pyruvate kinase was highly unusual and contrary to the typical effect of anoxia on pyruvate kinase in marine molluscs which is to stimulate the phosphorylation of pyruvate kinase and, thereby, convert the enzyme to a less active form.
Similar content being viewed by others
Abbreviations
- AABS :
-
p-(p-aminophenylazo)benzene sulphonic acid
- F2, 6P :
-
fructose-2,6-bisphosphate
- F6P :
-
fructose-6-phosphate
- G6P :
-
glucose-6-phosphate
- GP :
-
glycogen phosphorylase
- GS :
-
glycogen synthase
- I 50 :
-
inhibitor concentration reducing enzyme velocity by 50%
- MR :
-
metabolic rate
- PDH :
-
pyruvate dehydrogenase
- PEP :
-
phosphoenopyruvate
- PFK :
-
phosphofructokinase
- PK :
-
pyruvate kinase
- SW :
-
sea water
- F a :
-
air temperature
- TCA :
-
trichloroacetic acid
- UDPG :
-
uridine-diphosphate glucose
- WW :
-
wet weight
References
Aarset AV (1982) Freezing tolerance in intertidal invertebrates (a review). Comp Biochem Physiol 73A: 571–580
Brooks SPJ (1992) A simple computer program with statistical tests for the analysis of enzyme kinetics. Biotechniques 13: 906–911
Brooks SPJ, Storey KB (1992) Properties of pyruvate dehydrogenase from the land snail, Otala lactea: control of enzyme activation during estivation. Physiol Zool 63: 620–633
Cohen P (1980) Recently discovered systems of enzyme regulation by reversible phosphorylation. Elsevier/North Holland Biochemical Press, Amsterdam
De Vooys CGN, Holwerda DA (1986) Anaerobic metabolism in sublittoral living Mytilus galloprovincialis Lam. in the Mediterranean-III. The effect of anoxia and osmotic stress on some kinetic parameters of adductor muscle pyruvate kinase. Comp Biochem Physiol 85B: 217–221
Ebberink RHM, Salimans M (1982) Control of glycogen phosphorylase activity in the posterior adductor sea mussel Mytilus edulis. J Comp Physiol B 148: 27–33
Famme P, Knudsen J, Hansen ES (1981) The effect of oxygen on the aerobic-anaerobic metabolism of the marine bivalve, Mytilus edulis. Mar Biol Lett 2: 345–351
Gabbott PA, Cook PA, Whittle MA (1979) Seasonal changes in glycogen synthetase activity in the mantle tissue of Mytilus edulis L.: regulation by tissue glycogen. Biochem Soc Trans 7: 895–896
Gabbott PA, Whittle MA (1986) Glycogen synthetase in the sea mussel Mytilus edulis L. — II. Seasonal changes in glycogen content and glycogen synthetase activity in the mantle tissue. Comp Biochem Physiol 83B: 197–207
Gilles R (ed) (1979) Mechanisms of osmoregulation in animals. Wiley-Interscience, New York
Holwerda DA, Veenhof PR, Van Heugten HAA, Zandee DI (1983) Modification of mussel pyruvate kinase during anaerobiosis and after temperature acclimation. Molec Physiol 3: 225–234
Kanwisher JW (1955) Freezing in intertidal animals. Biol Bull 109: 56–63
Klutymans JH, de Bont AMT, Kruitwagen ECJ, Ravestein HJL, Veenhof PR (1983) Anaerobic capacities and anaerobic energy production of some Mediterranean bivalves. Comp Biochem Physiol 75B: 171–179
Livingstone DR, Zwaan A de (1983) Carbohydrate metabolism in gastropods. In: Wilbur KM (ed) The Mollusca. Academic Press, New York, pp 177–242
Loomis SH (1987) Freezing in intertidal invertebrates: an update. Cryo-Lett 8: 186–195
Michaelidis B, Gaitanki C, Beis I (1988) Modification of pyruvate kinase from the foot muscle of Patella caerulea (L) during anaerobiosis. J Exp Zool 248: 264–271
Murphy DJ (1983) Freezing resistance in intertidal invertebrates. Annu Rev Physiol 45: 289–299
Murphy DJ, Johnson LC (1980) Physical and temporal factors influencing the freezing tolerance of the marine snail Littorina littorea (L.). Biol Bull 158: 220–232
Plaxton WC, Storey KB (1984) Purification and properties of pyruvate kinase from red muscle tissue of the channelled whelk Busycotypus canaliculatum. Eur J Biochem 143: 257–265
Plaxton WC, Storey KB (1986) Glycolytic enzyme binding and metabolic control in anaerobiosis. J Comp Physiol 156B: 635–640
Seibenaller JF (1979) Regulation of pyruvate kinase in Mytilus edulis by phosphorylation-dephosphorylation. Mar Biol Lett 1: 105–110
Shick JM, Zwaan A de, de Bont AM (1983) Anoxic metabolic rate in the mussel Mytilus edulis L. estimated by simultaneous direct calorimetry and biochemical analysis. Physiol Zool 56: 56–63
Storey KB (1988) Mechanisms of glycolytic control during facultative anaerobiosis in a marine mollusc: tissue-specific analysis of glycogen phosphorylase and fructose-2,6-bisphosphate. Can J Zool 66: 1767–1771
Storey KB (1993) Molecular mechanisms of metabolic arrest in mollusks. In: Hochachka PW et al (eds) Surviving hypoxia: mechanisms of control and adaptation. CRC Press, Boca Raton, pp 253–269
Storey KB, Storey JM (1990) Facultative metabolic rate depression: molecular regulation and biochemical adaptation in anaerobiosis, hibernation, and estivation. Q Rev Biol 65: 145–174
Vaquez-Baanante I, Rosell-Perez M (1979) In vitro studies of Pecten maximus glycogen phosphorylase and the interconversion of their forms. Comp Biochem Physiol 62B: 381–387
Wieser W (1980) Metabolic end products in three species of marine gastropods. J Mar Biol Assoc UK 60: 175–180
Whitwan RE, Storey KB (1990a) Organ-specific analysis of the time-course of covalent modification of pyruvate kinase during anaerobiosis in a marine whelk. Physiol Zool 63: 222–234
Whitwam RE, Storey KB (1990b) Pyruvate kinase from the land snail Otala lactea: regulation by reversible phosphorylation during estivation and anoxia. J Exp Biol 154: 321–337
Whitwam RE, Storey KB (1991) Regulation of phosphofructokinase during estivation and anoxia in the land snail, Otala lactea. Physiol Zool 64: 595–610
Woodford TA, Taylor SJ, Corbin JD (1992) The biological functions of protein phosphorylation and dephosphorylation. In: Bittar E (ed) Fundementals of medical cell biology, vol 3B. JAI Press, Greenwich, CT, pp 453–607
Zwaan A de (1983) Carbohydrate catabolism in bivalves. In: Wilbur KM (ed) The Mollusca. Academic Press. New York, pp 137–175
Zwaan A de, Cortesi P, van den Thillart G, Roos J, Storey KB (1991) Differential sensitivities to hypoxia by two anoxiatolerant marine molluscs: a biochemical analysis. Mar Biol 111: 343–351
Author information
Authors and Affiliations
Additional information
Communicated by L.C.-H. Wang
Rights and permissions
About this article
Cite this article
Russell, E.L., Storey, K.B. Anoxia and freezing exposures stimulate covalent modification of enzymes of carbohydrate metabolism in Littorina littorea . J Comp Physiol B 165, 132–142 (1995). https://doi.org/10.1007/BF00301477
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00301477