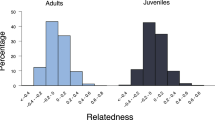
Summary
Variability at seven polymorphic allozyme loci and observations of dispersal and mating provide evidence for nonrandom genetic structure among adult female groups of the highly social bat, Desmodus rotundus. The average degree of relatedness, estimated by allelic correlations at each locus, within three and six groups of females is 0.018 (SE=0.013) and 0.032 (SE=0.023), respectively. Even though these estimates do not differ significantly from zero, a multivariate analysis of variance of individual allele frequencies reveals that three of six pairwise comparisons of groups reach significance. This genetic heterogeneity within a population does not lead to increased genetic subdivision between populations. Mean classificatory ability of the discriminant functions drops from 84% for assignment to group to 56% for assignment to population. This pattern of genetic variability is due to recruitment of female offspring into their natal groups and forced male dispersal. Occasional movements of unrelated females between groups lead to the formation of multiple matrilines within groups. Although males fight viciously for access to the top of preferred female roosting sites and top males mate preferentially with females in that roost, mean maximum paternity for top males is only 46%. Consequently, male mating success is sufficiently random to maintain gametic equilibria among all pairs of loci. Given an infant mortality of 54%, mean male tenure of 17 months, and a birth interval of 10 months, females are unlikely to be related through common male ancestors. In one group, the average degree of relatedness, derived from matrilineal pedigrees, is 0.11 (SD=0.17). Computer simulations of the growth of a group of female D. routundus show that the low level of relatedness within groups is expected even if the proportion of unrelated females allowed into a group decreases. This pattern holds for any animal which recruits one sex into its social group and has relatively high juvenile mortality followed by low adult mortality.
Similar content being viewed by others
References
Boorman SA, Levitt PR (1980) The genetics of altruism. Academic Press, New York
Boyd R, Richardson PJ (1980) Effect of phenotypic variation on kin selection. Proc Natl Acad Sci USA 77:7506–7509
Brown JS, Sanderson MJ, Michod RE (1982) Evolution of social behavior by reciprocation. J Theor Biol 99:319–339
Bush GL, Case SM, Wilson AC, Patton JL (1977) Rapid speciation and chromosomal evolution in mammals. Proc Natl Acad Sci USA 74:3942–3946
Chakraborty R (1982) Allocation versus variation: the issue of genetic differences between human racial groups. Am Nat 120:403–404
Charlesworth B (1979) A note on the evolution of altruism in structured demes. Am Nat 111:1010–1014
Chesser RK (1983) Genetic variability within and among populations of the black-tailed prairie dog. Evolution 37:320–331
Craig R, Crozier RH (1979) Relatedness in the polygynous ant Mymecia pilosula. Evolution 31:335–341
Daly JC (1981) Effects of social organization and environmental diversity on determining the genetic structure of a population of the wild rabbit, Oryctolagus cuniculus. Evolution 35:689–706
De Groot MH, Li CC (1960) Simplified method of estimating the MNS gene frequencies. Ann Hum Genet 24:109–115
Eshel I, Cavalli-Sforza LL (1982) Assortment of encounters and evolution of cooperativeness. Proc Natl Acad Sci USA 79:1331–1335
Falconer DS (1981) Introduction to quantitative genetics. Longman, New York
Fleming TH, Hooper ET, Wilson DE (1972) Three central american bat communities: structure, reproductive cycles, and movement patterns. Ecology 53:555–569
Foltz DW, Hoogland JL (1981) Analysis of the mating system in the black-tailed prairie dog (Cynomys ludovicianus) by likelihood of paternity. J Mammal 62:706–712
Foltz DW, Hoogland JL (1983) Genetic evidence of outbreeding in the black-tailed prairie dog (Cynomys ludovicianus). Evolution 37:273–281
Frankie GW, Baker HG, Opler PA (1974) Comparative phenological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. J Ecol 62:881–919
Hamilton WD (1964) The genetical evolution of social behavior. J Theor Biol 7:1–52
Hankin J, Sherman P (1981) Multiple paternity in Belding's ground squirrel litters. Science 212:351–353
Harris H, Hopkinson DA (1978) Handbook of enzyme electrophoresis in human genetics. American Elsevier, New York
Hedrick PW (1975) Genetic similarity and distance: comments and comparisons. Evolution 29:362–366
Hoogland JL (1983) Black-tailed prairie dog coteries are cooperatively breeding units. Am Nat 121:275–280
Janzen DH (1983) Costa rican natural history. University of Chicago Press, Chicago, Ill
Lester LJ, Selander RK (1981) Genetic relatedness and the social organization of Polistes colonies. Am Nat 117:147–166
McCracken GF (1984) Social dispersion and genetic variation in two species of emballonurid bats. Z Tierpsychol 66:55–69
McCracken GF, Bradbury JW (1977) Paternity and genetic heterogeneity in the polygynous bat, Phyllostomus hastatus. Science 198:303–306
McCracken GF, Bradbury JW (1981) Social organization and kinship in the polygynous bat, Phyllostomus hastatus. Behav Ecol Sociobiol 8:11–34
Metcalf RA, Whitt GS (1977) Intra-nest relatedness in the social wasp Polistes metricus. Behav Ecol Sociobiol 2:339–351
Nei M (1977) F-statistics and analysis of gene diversity in subdivided populations. Am Hum Genet 41:225–233
Nie NH, Hull CH, Jenkins JG, Steinbrenner K, Bent DH (1975) Statistical package for the social sciences, 2nd edn. McGraw Hill, New York
Olivier TJ, Ober C, Buettner-Janusch J, Sade DS (1981) Genetic differentiation among matrilines in social groups of rhesus monkeys. Behav Ecol Sociobiol 8:279–285
Packer C (1979) Inter-troop transfer and inbreeding avoidance in Papio anubis. Anim Behav 27:1–36
Pamilo P (1981) Genetic organization of Formica sanguinea populations. Behav Ecol Sociobiol 9:45–50
Pamilo P (1982) Genetic population structure in polygynous Formica ants. Heredity 48:95–106
Pamilo P (1983) Genetic differentiation within subdivided populations of Formica ants. Evolution 37:1010–1022
Pamilo P (1984) Genotypic correlation and regression in social groups: multiple alleles, multiple loci and subdivided populations. Genetics 107:307–320
Pamilo P, Crozier RH (1982) Measuring genetic relatedness in natural populations: methodology. Theor Popul Biol 21:171–193
Pamilo P, Varvio-Aho S (1979) Genetic structure of nests in the ant Formica sanguinea. Behav Ecol Sociobiol 6:91–98
Patton JL, Feder JH (1981) Microspatial genetic heterogeneity in pocket gophers: nonrandom breeding and drift. Evolution 35:912–920
Pearson B (1982) Relatedness of normal queens (macrogynes) in nests of the polygynous ant Myrmecia rubra Latreille. Evolution 36:107–112
Rogers JS (1972) Measures of genetic similarity and genetic distance. Studies in genetics VII. Univ Texas Publ 7213:145–153
Sassaman C (1978) Mating systems in porcellionid isopods multiple paternity and sperm mixing in Porcellio scaber Latr Heredity 41:385–397
Schmidt U (1974) Die Tragezeit der Vampirfledermause (Desmodus rotundus). Z Saugetierkd 39:129–132
Schmidt U (1978) Vampifledermause. Ziemsen, Wittenburg
Schmidt U, Schmidt C, Lopez-Forment W, Crespo RF (1978) Banding experiment on vampire bats (Desmodus rotundus) in Mexico. Z Saugetierkd 43:70–75
Schwartz OA, Armitage KB (1980) Genetic variation in social mammals: the marmot model. Science 207:665–667
Selander RK, Smith MH, Yang SV, Johnson WE, Gentry JB (1971) Biochemical polymorphism and systematics in the genus Peromyscus. I. Variation in the old field mouse. Studies in genetics VI. Univ Texas Publ 7103:49–90
Smouse PE, Spielman RS, Park MH (1982) Multiple-locus allocation of individuals to groups as a function of the genetic variation within and differences among human populations. Am Nat 119:445–463
Sokal RR, Rohlf FJ (1981) Biometry. Freeman, San Fransisco, Calif
Spiess EB (1977) Genes in populations. Wiley, New York
Stanton RG (1960) Genetic correlations with multiple alleles. Biometrics 16:235–244
Turner DC (1975) The vampire bat. Johns Hopkins University Press, Baltimore, Md
Wade MJ (1978) A critical review of the models of group selection. Q Rev Biol 53:101–114
Wade MJ (1980) Kin selection: its components. Science 210:665–667
Ward PS (1983) Genetic relatedness and colony organization in a species complex of ponerine ants. I. Phenotypic and genotypic composition of colonies. Behav Ecol Sociobiol 12:285–299
Weir BS, Cockerham CC (1978) Testing hypotheses about linkage disequilibrium with multiple alleles. Genetics 88:633–842
Wilkinson GS (1984) Reciprocal food sharing in vampire bats. Nature 308:181–184
Wilkinson GS (1985) The social organization of the common vampire bat. I. Pattern and cause of association. Behav Ecol Sociobiol 17:111–121
Wilson DS (1980) The natural selection of populations and communities. Cummings, Menlo Park, Calif
Wright S (1968) Evolution and the genetics of populations, vol 1. University of Chicago Press, Chicago, Ill
Wright S (1978) Evolution and the genetics of populations, vol 4. University of Chicago Press, Chicago, Ill
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilkinson, G.S. The social organization of the common vampire bat. Behav Ecol Sociobiol 17, 123–134 (1985). https://doi.org/10.1007/BF00299244
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00299244