Abstract
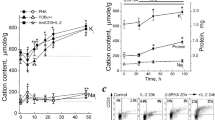
The level of intracellular glutathione (GSH) in mitogen-stimulated mouse lymphocytes is increased in the presence of 2-mercaptoethanol (2-ME), an enhancer of lymphocyte activation and proliferation. Since proliferation of lymphocytes in response to mitogens involves direct activation by a mitogen followed by continued proliferation in response to interleukin-2 (IL-2), we have investigated the effect of 2-ME and exogenous IL-2 on the GSH content and cell proliferation of rat lymphocytes stimulated with phytohemagglutinin (PHA). PHA stimulation increased both GSH content and the magnitude of the proliferative response, as measured by thymidine incorporation into cellular DNA. However, incubation of stimulated lymphocytes with 2-ME or IL-2 for 72 hr produced a significant further elevation of GSH levels and thymidine incorporation. 2-ME also increased the GSH content in unstimulated cultures, but it had little effect on thymidine incorporation. IL-2 increased GSH content and decreased thymidine incorporation in unstimulated lymphocytes. Exposure of cells to DL-buthionine-(S,R)-sulfoximine (BSO), an inhibitor of GSH biosynthesis, significantly depleted GSH and lowered the proliferative response, suggesting a crucial role of de novo GSH synthesis for lymphocyte activation. The data suggest that both 2-ME and IL-2 promote lymphocyte proliferation, although the mechanisms by which intracellular GSH levels are increased by the agents are apparently different.
Similar content being viewed by others
References
AIDOO, A., LYN-COOK, L.E., and CASCIANO, D.A. (1990). “Modulation of mitogenic activation of lymphocytes by antioxidants.’ Environ. Mol. Mutagenesis. 15, Supplement (17):6.
AIDOO, A., MORRIS, S.M., DOMON, O.E., MCGARRITY, L.J., KODELL, R.L., and CASCIANO, D.A. (1989). “Modulation of SCE induction and cell proliferation by 2-mercaptoethanol in phytohemagglutinin-stimulated rat lymphocytes.” Cell Biol. Toxicol. 5:237–248.
BANNAI, S. (1984). “Induction of cystine and glutamate transport activity in human fibroblast by diethylmaleate and other eletrophilic agents.” J. Biol. Chem. 259:2435–2440.
BANNAI, S. and ISHII, T. (1982). “Transport of cystine and cysteine and cell growth in cultured human diploid fibroblasts: effects of glutamate and homocysteate.” J. Cell Physiol. 112:6246–6249.
BUDROE, J.D., SHADDOCK, J.G., and CASCIANO, D.A. (1984). “A study of the potential genotoxicity of methapyrilene and related antihistamines using the hepatocyte/DNA repair assay.” Mutation Res. 135:131–137.
CANTRELL, D.A. and SMITH, K.A. (1984). “The interleukin-2-T-cell system: A new cell growth model.” Science 224:1312–1316.
DOYLE, M.V., LEE, M.T., and FONG, S. (1985). “Comparison of the Biological Activities of Human Recombinant Interleukin-2125 and Native interleukin-2.” J. Biol. Resp. Modifiers. 4:96–109.
FARRAR, J.J., BENJAMIN, W.R., HILFIKER, M.L., HOWARD, M., FARRAR, J.F. (1982). “The biochemistry, biology, and role of interleukin-2 in the induction of cytotoxic T cell and antibody-forming B cell responses.” Immunol. Rev. 63:129–166.
FIDELUS, R.K. (1988). “The generation of oxygen radicals: A positive signal for lymphocyte activation.” Cell. Immunol. 113:175–182.
FIDELUS, R.K., GINOUVES, P., LAWRENCE, D., and TSAN, M.F. (1987). “Modulation of intracellular glutathione concentration alters lymphocyte activation and proliferation.” Ecp. Cell Res. 170:269–275.
FIDELUS, R.K. and TSAN, M.F. (1986). “Enhancement of intracellular glutathione promotes lymphocyte activation by mitogen.” Cell. Immunol. 97:155–163.
FISCHMAN, C.M., UDEY, M.C., KURTZ, M., and WEDNER, H.J. (1981). “Inhibition of lectin-induced lymphocyte activation by 2-cyclohexene-1-one: Decrease intracellular glutathione inhibits an early event in the activation sequence.” J. Immunol. 127:2257–2262.
FONG, T.C. and MAKINODAN, T. (1989). “Preferential enhancement by 2-mercaptoethanol of IL-2 responsiveness of T blast cells from old over young mice is associated with potentiated protein kinase C translocation.” Immunol. Letters 20:149–154.
GRIFFITH, O.W., ANDERSON, M.E., and MEISTER, A. (1979). “Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine). a selective inhibitor of g-glutamylcysteine synthetase.” J. Biol. Chem. 254:1205–1210.
GRIMM, E.A., MASUMDER, A., ZHANG, H.Z., and ROSENBERG, S.A. (1982). “Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin-2-activated autologous human peripheral blood lymphocytes.” J. Exp. Med. 155:1823–1841.
HAMILOS, D.L. and WEDNER, H.J. (1985). “The role of glutathione in lymphocyte activation. I. Comparison of inhibitory effects of buthione sulfoximine and 2-cyclohexene-1-one by nuclear size transformation.” J. Immunol. 135:2740–2747.
ISHII, T., BANNAI, S., and SUGITA, Y. (1981). “Mechanism of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro: role of the mixed disulfide of 2-mercaptoethanol and cysteine. J. Biol. Chem. 256:12387–12392.
ISSELS, R.D., BOURIER, S., BIAGLOW, J.E., GERWECK, L.E., and WILMANNS, W. (1985). “Temperature-dependent influence of thiols upon glutathione levels in Chinese hamster ovary cells at cytotoxic concentrations.” Cancer Res. 45:6219–6224.
KANG, Y.-J. and ENGER, M.D. (1987). “Effect of cellular glutathione depletion on cadmium-induced cytoxicity in human lung carcinoma cells.” Cell Biol. Toxicol. 3:347–360.
KOLITZ, J.E., WONG, G.Y., WELTE, K., MERLUZZI, V.J., ENGERT, A., BIALAS, T., POLIVKA, A., BRADLEY, E.C., KONRAD, M., GNECCO, C., OETTGEN, H.F., and MERTLESMANN, R. (1988). “Phase I Trial of recombinant interleukin-2 and cyclophsohamide: Augmentation of cellular immunity and T-cell mitogenic response with long-term administration of rIL-2. J. Bio. Response Med. 7:457–472.
LIANG, C.M., LEE, N., CATELL, O., and LIANG, S.M. (1989). “Glutathione regulates interleukin-2 activity on cytotoxic T cells.” J. Biol. Chem. 264:13519–13523.
LOWRY, O.H., ROSEBROUGH, N.J., FARR, A.L., and RANDALL, R.J. (1951). “Protein measurement with the Folin phenol reagent.” J. Biol. Chem. 193:265–275.
MALKOVSKY, M., LOVELAND, B., NORTH, M., ASHERSON, G., GAO, L., WARD, P., and FIERS, W. (1987). “Recombinant interleukin-2 directly augments the cytotoxicity of human monocytes.” Nature 325:262–265.
MEISTER, A. and ANDERSON, M.E. (1983). “Glutathione.” Ann. Rev. Biochem. 52:711–760.
MORGAN, D.A., RUSCETTI, F.W., and GALLO, R.C. (1976). “Selective in vitro growth of T lymphocytes from normal bone marrow.” Science 193:1007–1008.
MORRIS, S.M., AIDOO, A., DOMON, O.E., MCGARRITY, L.J., KODELL, R.L., SCHOL, H.M., HINSON, W.G., PIPKIN, J.L., and CASCIANO, D.A. (1990). “Effect of interleukin-2 on cell proliferation, sister-chromatid exchange induction, and nuclear stress protein phosphorylation in PHA-stimulated Fischer 344 rat spleen lymphocytes: Modulation by 2-mercaptoethanol.” Environ. Mol. Mutagenesis. 15:10–18.
NOELLE, R.J. and LAWRENCE, D.A. (1980). “Modulation of T-cell functions. I. Effect of 2-mercaptoethanol and macrophages on T-cell proliferation.” Cell Immunol. 50:416–431.
ORTALDO, J.R., MASON, A.T., GERARD, J.P., HENDERSON, L.E., FARRAR, W., HOPKINS III, R.F., HERBERMAN, R.B., and RABIN, H. (1984). “Effects of natural and recombinant IL-2 on regulation of IFN-y production and natural killer activity: lack of involvement of the TAC antigen for these immunoregulatory effects.” J. Immunol. 133:779–783.
ROBB, R.J., MUNCK, A., and SMITH, K.A. (1981). “T cell growth factor receptors. Quantitation, specificity and biological relevance.” J. Exp. Med. 154:1455–1474.
ROSENBERG, S.A., LOTZE, M.T., MUUL, L.M., CHANG, A.E., AVIS, F.P., LEITMAN, S., LINEHAN, W.M., ROBERTSON, C.N., LEE, R.E., RUBIN, J.T., SEIPP, C.A., SIMPSON, C.G., and WHITE, D.E. (1987). “Progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone.” New Eng. J. Med. 316:889–897.
RUSCETTI, F.W., MORGAN, D.A., and GALLO, R.C. (1977). “Functional and morphorlogic characterization of human T-cells continuously grown in vitro.” J. Immunol. 119:131–138.
SHAW, J., MONTICONE, V., and PAETKAU, V. (1978). “Partial purification and molecular characterization of a lymphokine (co-stimulator) required for the mitogenic response of mouse thymocytes in vitro.” J. Immuno. 120: 1967–1973.
SMITH, K.A. (1984). “Interleukin-2.” Annu. Rev. Immunol. 2:319–333.
STOTTER, H., RUDE, E., and WAGNER, H. (1980). “T cell factor (interleukin-2) allows in vivo induction of T helper cells against heterologous erythrocytes in athymic (nu/nu) mice.” Eur. J. Immunol. 10: 719–722.
SUTHANTHIRAN, M., ANDERSON, M.E., SHARMA, V.K., and MEISTER, A. (1990). “Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens.” Proc. Natl. Acad. Sci. USA. 87:3343–3347.
TETEISHI, N., HIGASCHI, T., NATUSE, A., HIKITA, K., and SAKAMOTO, Y. (1981). “Relative contributions of sulfure atoms of dietary cysteine and methionine to rat liver glutathione and proteins.” J. Biochem. 90:1603–1610.
TIETZE, F. (1969). “Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues.” Annal. Biochem. 27:502–522.
TSUDO, M., UCHIYAMA, T., and UCHINNO, H. (1984). “Expression of Tac antigen on activated normal human B cells.” J. Exp. Med. 160:612–617.
WAGNER, H., HARDT, C., HEEG, K., ROLLINGHOFF, M., and PFIZENMALER, K. (1980). “T cell derived helper factor allows in vivo induction of cytotoxic in nu/nu mice.” Nature 284:278–279.
WILLIAMSON, J.M., BOETTCHER, B., and MEISTER, A. (1982). “Intracellular cysteine delivery system that protects against toxicity by promoting glutathione synthesis.” Proc. Natl. Acad. Sci. USA. 79:6246–6249.
WILLIAMSON, J.M. and MEISTER, A. (1981). “Stimulation of hepatic glutathione formation by adminstration of L-2-oxothiazolidine-4-carboxylate, a 5-oxo-L-prolinase substrate.” Proc. Natl. Acad. Sci. USA. 78:936–939.
ZMUDA, J. and FRIEDENSON, B. (1983). “Changes in intracellular glutathione levels in stimulated and unstimulated lympyocytes in the presence of 2-mercaptoethanol or cysteine.” J. Immunol. 130:362–364.
Author information
Authors and Affiliations
Additional information
Copies of articles are available through ISI Document Delivery Services c/o The Genuine Article, 3501 Market Street, Philadelphia, PA 19104.
Rights and permissions
About this article
Cite this article
Aidoo, A., Lyn-Cook, L.E., Morris, S.M. et al. Comparative study of intracellular glutathione content in rat lymphocyte cultures treated with 2-mercaptoethanol and interleukin-2. Cell Biol Toxicol 7, 215–227 (1991). https://doi.org/10.1007/BF00250976
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00250976