Summary
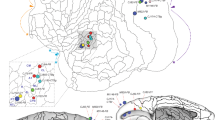
An attempt was made to correlate electrophysiological and morphological characteristics of rat ventral cochlear nucleus neurons. Their axonal course and their soma morphology were investigated using the intra-axonal horseradish peroxidase method. Prior to labeling, neurons were characterized by recording their response patterns to acoustic stimulation with pure tones. Three types of cells were found: Category I (37 neurons) exhibited “primarylike” responses and a spontaneous firing rate below 10 spikes/s. Category II (21 neurons) showed “on” responses and little spontaneous activity. Category III (9 neurons) had “primarylike” responses like neurons in category I. However, the spontaneous activity rate of these neurons was significantly higher (mean: 95 spikes/s). Among the response categories, the morphological characteristics differed in some prominent aspects. Within each category, however, the morphological properties were rather similar. All neurons in category I were globular/bushy cells located in the area of the entrance of the cochlear nerve. The axon of each cell coursed along the ventral acoustic stria and consistently innervated the lateral superior olive ipsilaterally, and the nucleus of the trapezoid body and the nucleus of the lateral lemniscus contralaterally. Some neurons also projected to periolivary nuclei ipsilaterally and contralaterally. Neurons in category II were located in the posteroventral cochlear nucleus and were presumably multipolar/stellate cells. Their axons coursed via the intermediate acoustic stria and innervated mainly contralateral periolivary regions as well as the contralateral nucleus of the lateral lemniscus. Ipsilaterally, the lateral superior olive and the superior periolivary nucleus were innervated by some of the category II neurons. Somata types of neurons in category III could not be identified morphologically, but somata were located in caudal parts of the posteroventral cochlear nucleus that correspond to the octopus cell area. Their axons coursed via the intermediate acoustic stria and innervated periolivary regions and the contralateral nucleus of the lateral lemniscus. Thus, their axonal distribution differed only slightly from neurons in category II. These data confirm and extend previous findings regarding the efferent connections of ventral cochlear neurons. They emphasize the complexity of the axonal projection patterns of single cochlear nucleus cells. Since two types of response patterns and three types of axonal projection patterns have been observed, there remains an ambiguous relation between response pattern and axonal projection site. It is concluded that the response pattern to pure tone stimulation alone is not sufficient to describe physiological characteristics allowing for the establishment of structure-function relations. Only if one considers additional physiological properties like the spontaneous activity rate and the shape of tuning curves, does a correspondence between structure and function become apparent.
Similar content being viewed by others
Abbreviations
- AVCN:
-
anteroventral cochlear nucleus
- c:
-
contralateral
- CF:
-
characteristic frequency
- CN:
-
cochlear nucleus
- DCN:
-
dorsal cochlear nucleus
- dor:
-
dorsal
- DPO:
-
dorsal periolivary region
- HRP:
-
horseradish peroxidase
- i:
-
ipsilateral
- IAS:
-
intermediate acoustic stria
- IC:
-
inferior colliculus
- lat:
-
lateral
- LL:
-
lateral lemniscus
- LNTB:
-
lateral nucleus of the trapezoid body
- LSO:
-
lateral superior olive
- LVPO:
-
lateroventral periolivary nucleus
- med:
-
medial
- MNTB:
-
medial nucleus of the trapezoid body
- MSO:
-
medial superior olive
- MVPO:
-
medioventral periolivary nucleus
- NLL:
-
nucleus of the lateral lemniscus
- NTB:
-
nucleus of the trapezoid body
- PSTH:
-
peristimulus time histogram
- PVCN:
-
posteroventral cochlear nucleus
- RF:
-
reticular formation
- rost:
-
rostral
- RPO:
-
rostral periloivary region
- SOC:
-
superior olivary complex
- SPO:
-
superior paraolivary nucleus
- VAS:
-
ventral acoustic stria
- VCN:
-
ventral cochlear nucleus
- VNLL:
-
ventral nucleus of the lateral lemniscus
- VNTB:
-
ventral nucleus of the trapezoid body
- 8n:
-
cochlear nerve
References
Adams JC (1979) Ascending projections to the inferior colliculus. J Comp Neurol 183: 519–538
Adams JC (1981) Heavy metal intensification of DAB-based reaction product. J Histochem Cytochem 29: 775
Aitkin LM, Anderson DJ, Brugge JF (1970) Tonotopic organization and discharge characteristics of single neurons in nuclei of the lateral lemniscus of the cat. J Neurophysiol 33: 421–440
Borg E (1973) A neuroanatomical study of the brainstem auditory system of the rabbit, Part 1. Ascending connections. Acta Morphol Neerl Scand 11: 31–48
Boudreau JC, Tsuchitani C (1970) Cat superior olive S-segment cell discharge to tonal stimulation. In: Neff WD (ed) Auditory system, Vol 1. Contributions to sensory physiology, Vol IV. Academic, New York, pp 143–213
Bourk TR (1976) Electrical responses of neural units in the anteroventral cochlear nucleus of the cat. PhD thesis, MIT, Cambridge
Bourk TR, Mielcarcz JP, Norris BE (1981) Tonotopic organization of the anteroventral cochlear nucleus of the cat. Hear Res 4: 215–241
Brawer JR, Morest DK, Kane EC (1974) The neuronal architecture of the cochlear nucleus of the cat. J Comp Neurol 155: 251–299
Britt RH, Rossi GT, Morest DK (1983) Intracellular studies in cat cochlear nucleus: correlation of physiological responses and morphology of intracellularly labelled cat cochtear nucleus neurons. In: Webster WR, Aitkin LM (eds) Mechanisms of hearing. Monash Univ Press, Clayton, p 125
Brown AG, Fyffe REW (1984) Intracellular staining of mammalian neurons. Academic Press, London
Brownell WE (1975) Organization of cat trapezoid body and the discharge characteristics of its fibers. Brain Res 94: 413–433
Caird D, Klinke R (1983) Processing of binaural stimuli by cat superior olivary complex neurons. Exp Brain Res 52: 385–399
Cant NB (1984) The fine structure of the lateral superior olivary nucleus of the cat. J Comp Neurol 227: 63–77
Cant NB, Casseday JH (1986) Projections from the anteroventral cochlear nucleus to the lateral and medial superior olivary nuclei. J Comp Neurol 247: 457–476
Cant NB, Morest DK (1979) Organization of the neurons in the anterior division of the anteroventral cochlear nucleus of the cat. Light-microscopic observations. Neurosci 4: 1909–1923
Covey E, Casseday JH (1986) Connectional basis for frequency representation in the nuclei of the lateral lemniscus of the bat Eptesicus fuscus. J Neurosci 6: 2926–2940
Elverland HH (1978) Ascending and intrinsic projections of the superior olivary complex in the cat. Exp Brain Res 32: 117–134
Evans EF, Nelson PG (1973) The responses of single neurons in the cochlear nucleus of the cat as a function of their location and anaesthetic state. Exp Brain Res 17: 402–427
Friauf E (1986) Morphology of motoneurons in different subdivisions of the rat facial nucleus stained intracellularly with horseradish peroxidase. J Comp Neurol 253: 231–241
Glendenning KK, Brunso-Bechtold JK, Thompson GC, Masterton RB (1981) Ascending auditory afferents to the nuclei of the lateral lemniscus. J Comp Neurol 197: 673–703
Glendenning KK, Hutson KA, Nudo RJ, Masterton RB (1985) Acoustic chiasm II: anatomical basis of binaurality in lateral superior olive of cat. J Comp Neurol 232: 261–275
Glendenning KK, Masterton RB (1983) Acoustic chiasm: efferent projections of the lateral superior olive. J Neurosci 3: 1521–1537
Godfrey DA, Kiang NYS, Norris BE (1975) Single unit activity in the posteroventral cochlear nucleus of the cat. J Comp Neurol 162: 247–268
Goldberg JM, Brownell WE (1973) Discharge characteristics of neurons in anteroventral and dorsal cochlear nuclei of cat. Brain Res 64: 35–54
Guinan JJ Jr, Guinan SS, Norris BE (1972a) Single auditory units in the superior olivary complex. I. Response to sounds and classifications based on physiological properties. Int J Neurosci 4: 101–120
Guinan JJ Jr, Norris BE, Guinan SS (1972b) Single auditory units in the superior olivary complex: II. Locations of unit categories and tonotopic organization. Int J Neurosci 4: 147–166
Harrison JM, Feldman ML (1970) Anatomical aspects of the cochlear nucleus and superior olivary complex. In: Neff WD (ed) Auditory system 1. Contributions to sensory physiology. Vol IV. Academic, New York, pp 95–142
Harrison JM, Irving R (1965) The anterior ventral chochlear nucleus. J Comp Neurol 124: 15–42
Harrison JM, Irving R (1966a) Ascending connections of the anterior ventral cochlear nucleus in the rat. J Comp Neurol 126: 51–64
Harrison JM, Irving R (1966b) The organization of the posterior ventral cochlear nucleus in the rat. J Comp Neurol 126: 391–402
Harrison JM, Warr WB (1962) A study of the cochlear nuclei and ascending auditory pathways of the medulla. J Comp Neurol 119: 341–380
Henkel CK, Spangler KM (1983) Organization of the efferent projections of the medial superior olivary nucleus in the cat as revealed by HRP and autoradiographic tracing methods. J Comp Neurol 221: 416–428
Irvine DRF (1986) The auditory brainstem. In: Ottoson D (ed) Progress in sensory physiology, Vol VII. Springer, Berlin Heidelberg New York
Itoh KA, Konishi S, Nomura N, Mizuno N, Nakamura Y, Sugimoto T (1979) Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase, cobalt-glucose method. Brain Res 175: 341–346
Kelly JB, Masterton RB (1977) Auditory sensitivity of the albino rat. J Comp Physiol Psychol 91: 930–936
Kiang NYS, Morest DK, Godgrey DA, Guinian JJ Jr, Kane EC (1973) Stimulus coding at caudal levels of the cat's auditory nervous system. I. Response characteristics of single neurons. In: Moller AR (ed) Basic mechanisms in hearing. Academic Press, New York, pp 455–478
Kudo M (1981) Projections of the nuclei of the lateral lemniscus in the cat: an autoradiographic study. Brain Res 221: 57–69
Lenn NJ, Reese TS (1966) The fine structure of nerve endings in the nucleus of the trapezoid body and the ventral cochlear nucleus. Am J Anat 118: 375–389
Lorente de Nó R (1933) Anatomy of the eighth nerve. III. General plan of structure of the primary cochlear nuclei. Laryngoscope 43: 327–350
Lorente de Nó R (1981) The primary acoustic nuclei. Raven, New York
Morest DK (1968a) The collateral system of the medial nucleus of the trapezoid body of the cat, its neuronal architecture and relation to the olivo-cochlear bundle. Brain Res 9: 288–311
Morest DK (1968b) The growth of synaptic endings in the mammalian brain: a study of the calyces of the trapezoid body. Z Anat Entwgesch 127: 201–220
Müller M (1987) Funktionelle Organisation akustischer Kerngebiete im Hirnstamm der Wüstenrennmaus Pachyuromys duprasi: eine kombinierte neuroanatomische und elektrophysiologische Untersuchung. Dissertation, University Frankfurt
Osen KK (1969a) Cytoarchitecture of the cochlear nuclei in the cat. J Comp Neurol 136: 453–484
Osen KK (1969b) The intrinsic organization of the cochlear nuclei in the cat. Acta Otolaryngol (Stockh) 67: 352–359
Osen KK (1972) Projection of the cochlear nuclei on the inferior colliculus in the cat. J Comp Neurol 144: 355–372
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, Sydney Orlando San Diego New York
Pfeiffer RR (1966) Classification of response patterns of spike discharges for units in the cochlear nucleus: tone-burst stimulation. Exp Brain Res 1: 220–235
Rhode WS, Oertel D, Smith PH (1983a) Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat ventral cochlear nucleus. J Comp Neurol 213: 448–463
Rhode WS, Smith PH, Oertel D (1983b) Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat dorsal cochlear nucleus. J Comp Neurol 213: 426–447
Rhode WS, Smith PH (1986) Encoding timing and intensity in the ventral cochlear nucleus of the cat. J Neurophysiol 56: 261–286
Ritz LA, Brownell WE (1982) Single unit analysis of the posteroventral cochlear nucleus of the decerebrate cat. Neurosci 7: 1995–2010
Rouiller EM, Ryugo DK (1983) Morphology of physiologically defined neurons in the ventral cochlear nucleus of cat. Fed Proc 42: 2714
Rouiller EM, Ryugo DK (1984) Intracellular marking of physiologically characterized cells in the ventral cochlear nucleus of the cat. J Comp Neurol 225: 167–186
Scheibel ME, Scheibel AB (1974) Neuropil organization in the superior olive of the cat. Exp Neurol 43: 339–348
Shneiderman A, Henkel CK (1985) Evidence of collateral axonal projections to the superior olivary complex. Hear Res 19: 199–205
Smith PH, Rhode WS (1987) Characterization of HRP-labeled globular bushy cells in the cat anteroventral cochlear nucleus. J Comp Neurol 266: 360–375
Smith PH, Carney LH, Yin TCT (1987) Projections of globular bushy cells in the cat. Proc Soc Neurosci 13: 151.1. Abstract
Spangler KM, Warr WB, Henkel CK (1985) The projections of principal cells of the medial nucleus of the trapezoid body in the cat. J Comp Neurol 238: 249–262
Tolbert LP, Morest DK (1982) The neuronal architecture of the anteroventral cochlear nucleus of the cat in the region of the cochlear nerve root: Golgi and Nissl methods. Neuroscience 7: 3013–3030
Tolbert LP, Morest DK, Yurgelun-Todd DA (1982) The neuronal architecture of the anteroventral cochlear nucleus of the cat in the region of the cochlear nerve root: horseradish peroxidase labelling of identified cell types. Neuroscience 7: 3031–3052
Tsuchitani C, Boudreau JC (1966) Single unit analysis of cat superior olive S segment with tonal stimuli. J Neurophysiol 29: 684–697
Warr WB (1969) Fiber degeneration following lesions in the posteroventral cochlear nucleus of the cat. Exp Neurol 23: 140–155
Warr WB (1982) Parallel ascending pathways from the cochlear nucleus: neuroanatomical evidence of functional specialization. In: Neff WD (ed) Contributions to sensory physiology, Vol 7. Academic Press, New York, pp 1–38
Whitley JM, Henkel CK (1984) Topographical organization of the inferior colliculus projection and other connections of the ventral nucleus of the lateral lemniscus in the cat. J Comp Neurol 229: 257–270
Willard FH, Ryugo DK (1983) Anatomy of the central auditory system. In: Willott JF (ed) The auditory psychobiology of the mouse. Thomas, Springfield, pp 201–304
Wu SH, Oertel D (1984) Intracellular injection with horseradish peroxidase of physiologically characterized stellate and bushy cells in slices of mouse anteroventral cochlear nucleus. J Neurosci 4: 1577–1588
Young ED, Voigt HF (1982) Response properties of type II and type III units in dorsal cochlear nucleus. Hear Res 6: 153–169
Zook JM, Casseday JH (1985) Projections from the cochlear nuclei in the mustache bat, Pteronotus parnellii. J Comp Neurol 237: 307–324
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Friauf, E., Ostwald, J. Divergent projections of physiologically characterized rat ventral cochlear nucleus neurons as shown by intra-axonal injection of horseradish peroxidase. Exp Brain Res 73, 263–284 (1988). https://doi.org/10.1007/BF00248219
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248219