Summary
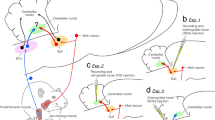
The cerebellar projection from the facial and motor trigeminal nuclei was studied in the cat by means of retrograde axonal transport of wheat germ agglutinin-horseradish peroxidase and fluorescent tracers. The feline facial nucleus was cytoarchitectonically subdivided into ventromedial, ventrolateral, lateral, dorsal, intermediate and medial divisions (see Papez 1927), and the motor trigeminal nucleus into medial, ventral, intermediate, lateral and dorsal divisions. The neurons in the facial and motor trigeminal nuclei were classified as small (ovoid to round cells with a maximum diameter of the cell body of about 20 μm) or large (polygonal to round cells with maximum diameter of about 40 μm). After floccular injections of the wheat germ agglutininhorseradish peroxidase complex, retrogradely labelled cells were found throughout the facial nucleus, but especially in its medial and dorsal divisions. In the motor trigeminal nucleus, labelled neurons were found only in the ventral, intermediate and lateral divisions. Cases with tracer deposition (implants or injections) in other parts of the cerebellar cortex or nuclei were all negative. All facial and motor trigeminal neurons labelled after floccular injections were smaller than the neurons labelled after injections in the facial mimic and masticatory muscles, and only single-labelled neurons were found following floccular injections of Fluoro-Gold and muscular injections of rhodamine-B-isothiocyanate in the same animals. These observations strongly suggest that the neurons in the facial and motor trigeminal nuclei which project to flocculus are of the non-motoneuron type.
Similar content being viewed by others
References
Batini C, Buisseret-Delmas C, Corvisier J (1976) Horseradish peroxidase localization of masticatory muscle motoneurons in cat. J Physiol (Paris) 72: 301–309
Berman AL (1968) The brain stem of the cat; a cytoarchitectonic atlas with stereotaxic coordinates. The University of Wisconsin Press, Madison Milwaukee London
Buskirk C van (1945) The seventh nerve complex. J Neurol 82: 303–333
Courville J (1966) The nucleus of the facial nerve: the relation between cellular groups and peripheral branches of the nerve. Brain Res 1: 338–354
Denavit-Saubié M, Corvisier J (1972) Cat trigeminal motor nucleus: rhythmic units firing in relation to opening movements of the mouth. Brain Res 40: 500–503
Dietrichs E, Walberg F (1983) Cerebellar cortical afferents from the red nucleus in the cat. Exp Brain Res 50: 353–538
Dietrichs E, Walberg F (1987) Cerebellar nuclear afferents —where do they originate? A re-evaluation of the projections from some lower brain stem nuclei. Anat Embryol 177: 165–172
Dietrichs E, Walberg F, Nordby T (1985) The cerebellar nucleoolivary and olivo-cerebellar nuclear projections in the cat as studied with anterograde and retrograde transport in the same animal after implantation of crystalline WGA-HRP. I. The dentate nucleus. Neurosci Res 3: 52–70
Gonatas NK, Harper C, Mizutani T, Gonatas JO (1979) Superior sensitivity of conjugates of horseradish peroxidase with wheat germ agglutinin for studies of retrograde axonal transport. J Histochem Cytochem 27: 728–734
Grant K, Guegan M, Horcholle-Bossavit G (1981) The anatomical relationship of the retractor bulbi and posterior digastric motoneurons to the abducens and facial nuclei in the cat. Arch Ital Biol 119: 195–207
Graybiel AM (1977) Organization of oculomotor pathways in the cat and Rhesus monkey. Dev Neurosci 1: 79–88
Hinrichsen CFL, Watson CD (1984) The facial nucleus of the rat: representation of facial muscles revealed by retrograde transport of horseradish peroxidase. Anat Rec 209: 407–415
Ito M (1984) The cerebellum and neural control. Raven Press, New York
Jacquin MF, Rhoades RW, Enfiejian HL, Egger MD (1983) Organization and morphology of masticatory neurons in the rat: a retrograde HRP study. J Comp Neurol 218: 239–256
Keller JT, Saunders MC, Ongkiko CM, Johnson J, Frank E, van Loveren H, Tew JM Jr (1983) Identification of motoneurons innervating the tensor tympani and tensor veli palatini muscles in the cat. Brain Res 270: 209–215
Konishi A, Yasui Y, Nomura S, Mizuno N, Sugimoto T, Itho K (1982) A quantitative electron microscope study of synaptic terminals on the tensor tympani motor neurons in the cat. Neuroscience [Suppl] 7: S119
Kotchabhakdi N, Walberg F (1977) Cerebellar afferents from neurons in motor nuclei of cranial nerves demonstrated by retrograde axonal transport of horseradish peroxidase. Brain Res 137: 158–163
Kume M, Uemura M, Matsuda K, Matsushima R, Mizuno N (1978) Topographical representation of peripheral branches of the facial nerve within the facial nucleus: a HRP study in the cat. Neurosci Lett 8: 5–8
Kuypers HGJM, Catsman-Berrevoets CE, Padt RE (1977) Retrograde axonal transport of fluorescent substances in the rat's forebrain. Neurosci Lett 6: 127–135
Langer T, Fuchs AF, Scudder CA, Chubb MC (1985) Afferents to the flocculus of the cerebellum in the rhesus macaque as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol 235: 1–25
Matsuda K, Uemura M, Takeuchi Y, Kume M, Matsushima R, Mizuno N (1979) Localization of motoneurons innervating the posterior belly of the digastric muscle: a comparative anatomical study by the HRP method. Neurosci Lett 12: 47–52
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117
Mizuno N, Konishi A, Sato M (1975) Localization of masticatory motoneurons in the cat and rat by means of retrograde axonal transport of horseradish peroxidase. J Comp Neurol 164: 105–116
Mizuno N, Nomura S, Konishi A, Uemura-Sumi M, Takahashi O, Yasui Y, Takada M, Matsushima R (1982) Localization of motoneurons innervating the tensor tympani muscles: an horseradish peroxidase study in the guinea pig and cat. Neurosci Lett 31: 205–208
Mori J, Hori N, Katsuda N (1981) A new method for application of horseradish peroxidase into a restricted area of the brain. Brain Res Bull 6: 19–22
Papez JW (1927) Subdivisions of the facial nucleus. J Comp Neurol 43: 159–191
Røste GK, Dietrichs E (1988a) The feline oculomotor nucleus: morphological subdivisions and projection to the cerebellar cortex and nuclei. Anat Embryol 178: 67–75
Røste GK, Dietrichs E (1988b) Cerebellar projecting cells in the facial and motor trigeminal nuclei — are they motoneurons? Eur J Neurosci [Suppl] p29
Sato Y, Kawasaki T, Ikarashi K (1983) Afferent projections from the brainstem to the three floccular zones in cats. II. Mossy fiber projections. Brain Res 272: 37–48
Schaefer K-P, Meyer DL, Schott D (1971) Optic and vestibular influences on ear movements. Brain Behav Evol 4: 323–333
Schmued LC, Fallon JH (1986) Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res 377: 147–154
Sessle BJ (1977) Identification of alpha and gamma trigeminal motoneurons and effects of stimulation of amygdala, cerebellum, and cerebral cortex. Exp Neurol 54: 303–322
Taber E (1961) The cytoarchitecture of the brain stem of the cat. J Comp Neurol 116: 27–70
Thanos S, Vidal-Sanz M, Aguayo AJ (1987) The use of rhodamine-B-isothiocyanate (RITC) as an anterograde and retrograde tracer in the adult rat visual system. Brain Res 406: 317–321
Xu Q, Grant G (1988) Do certain spinocerebellar neurons in lamina IX at lumbosacral levels send collaterals to peripheral nerves? A retrograde fluorescent double labeling study in the cat. Arch Ital Biol 126: 179–192
Walberg F, Nordby T, Dietrichs E (1987) The olivonodular projection: a re-examination based on folial cerebellar implants. Neurosci Lett 81: 82–88
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Røste, G.K. Non-motoneurons in the facial and motor trigeminal nuclei projecting to the cerebellar flocculus in the cat. Exp Brain Res 75, 295–305 (1989). https://doi.org/10.1007/BF00247935
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00247935