Summary
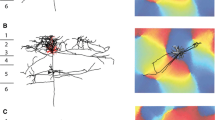
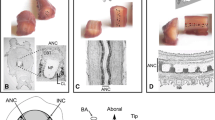
Immunocytochemical methods were used to study the distribution and ultrastructure of serotonin (5-hydroxytryptamine; 5-HT) immunoreactive fibers innervating the monkey sensory-motor cortex. Beaded 5-HT positive fibers were found in all cortical layers of both areas but with relatively fewer in middle cortical layers. Examination of 2 μm-thick plastic sections at the light microscope level, revealed that the vast majority of the bouton-like structures on the fibers lay in the neuropil and not adjacent to neuronal somata. A few beaded immunoreactive fibers were seen around certain pyramidal and nonpyramidal cell somata, very occasionally forming modest pericellular ramifications. Serial reconstructions made from electron micrographs after resectioning the 2 μm-thick sections, revealed that the dilatations of the fibers are 5-HT positive boutons but the boutons examined rarely formed morphologically identifiable synaptic contacts. Of 191 reconstructed boutons only 5 made contacts with obvious membrane specializations, all of which were of the asymmetrical type. No immunoreactive synaptic contacts were seen on pyramidal cell somata in the cortex, nor on dendrites or somata in the white matter underlying the cortex, although 5-HT positive boutons commonly lay closely adjacent to neuronal profiles in both sites. 5-HT fibers in the cortex and white matter have a similar morphological appearance and both myelinated and unmyelinated types are seen.
Similar content being viewed by others
References
Anden N-E, Dahlström A, Fuxe K, Larsson K (1965) Mapping of catecholamine and 5-hydroxytryptamine neurons innervating the telencephalon and diencephalon. Life Sci 4: 1275–1279
Anden N-E, Dahlström A, Fuxe K, Larsson K, Olson L, Ungerstedt U (1966) Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol Scand 67: 313–326
Beaudet A, Descarries L (1976) Quantitative data on serotonin nerve terminals in adult rat neocortex. Brain Res 111: 301–309
Beaudet A, Descarries L (1978) The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neuroscience 3: 851–860
Beaudet A, Descarries L (1984) Fine structure of monoamine axon terminals in cerebral cortex. In: Descarries L, Reader TA, Jasper HH (eds) Monoamine innervation of cerebral cortex. Alan R. Liss, New York, pp 77–93
Beaudet A, Sotelo C (1981) Synaptic remodeling of serotonin axon terminals in rat agranular cerebellum. Brain Res 206: 305–329
Cajal SR (1900) Estudios sobre la corteza cerebral humana: estructura de la corteza acústica. Rev Trimest Microgr 5: 129–183
Colonnier M (1968) Synaptic patterns on different cell types in the different laminae of the cat visual cortex: an electron microscope study. Brain Res 9: 268–287
DeFelipe J, Fairén A (1982) A type of basket cell in superficial layers of the cat visual cortex. A Golgi-electron microscope study. Brain Res 244: 9–16
DeFelipe J, Hendry SHC, Jones EG (1986) A correlative electron microscopic study of basket cells and large GABAergic neurons in the monkey sensory-motor cortex. Neuroscience 17: 991–1009
DeLima AD, Singer W (1987) The serotoninergic fibers in the dorsal lateral geniculate nucleus of the cat: distribution and synaptic connections demonstrated with immunocytochemistry. J Comp Neurol 258: 339–351
Descarries L, Beaudet A, Watkins KC (1975) Serotonin nerve terminals in adult rat neocortex. Brain Res 100: 563–588
Descarries L, Lapierre Y (1973) Noradrenergic axon terminals in the cerebral cortex of rat.I. Radioautographic visualization after topical application of DL-(3H) norepinephrine. Brain Res 51: 141–160
Descarries L, Watkins KC, Lapierre Y (1977) Noradrenergic axon terminals in the cerebral cortex of the rat. III. Topometric ultrastructural analysis. Brain Res 133: 197–222
Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63: 844–914
Foote SL, Morrison JH (1984) Postnatal development of laminar innervation patterns by monoaminergic fibers in monkey (Macaca fascicularis) primary visual cortex. J Neuroscience 4: 2667–2680
Fuxe K (1965) Evidence for the existence of monoamine neurons in the central nervous system. IV. Distribution of monoamine nerve terminals in the central nervous system. Acta Physiol Scand 64: 39–85
Fuxe K, Hamberger B, Hökfelt T (1968) Distribution of noradrenaline nerve terminals in cortical areas of the rat. Brain Res 8: 125–131
Gall C, Moore R (1984) Distribution of enkephalin, substance P, tyrosine hydroxylase, and 5-hydroxytryptamine immunoreactivity in the septal region of the rat. J Comp Neurol 225: 212–227
Hendry SHC, Schwark HD, Jones EG, Yan J (1987) Numbers and proportions of GABA immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci 7: 1503–1519
Jones EG (1986) Neurotransmitters in the cerebral cortex. J Neurosurg 65: 135–153
Jones EG, Hendry SHC (1984) Basket cells. In: Peters A, Jones EG (eds) Cerebral cortex, Vol 1. Plenum Press, New York, pp 309–336
Jones EG, Powell TPS (1970) Electron microscopy of the somatic sensory cortex of the cat. I. Cell types and synaptic organization. Philos Trans R Soc London Ser B 257: 1–11
Marin-Padilla M (1972) Double origin of the pericellular baskets of the pyramidal cells of the human motor cortex: a Golgi study. Brain Res 38: 1–12
Marin-Padilla M (1984) Neurons of layer I: a developmental analysis. In: Peters A, Jones EG (eds) Cerebral cortex, Vol 1. Cellular components of the cerebral cortex. Plenum Press, New York, pp 447–477
Molliver ME, Grzanna R, Lidov HGW, Morrison JH, Olschowka JA (1982) Monoamine systems in the cerebral cortex. In: Chan-Palay V, Palay SL (eds) Cytochemical methods in neuroanatomy. Alan R Liss, New York, pp 225–277
Moore RY, Bloom FE (1979) Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Ann Rev Neurosci 2: 113–168
Morrison JH, Foote SL (1986) Noradrenergic and serotoninergic innervation of cortical, thalamic and tectal visual structures in Old and New World monkeys. J Comp Neurol 243: 117–138
Morrison JH, Foote SL, Molliver ME, Bloom FE, Lidov HGW (1982) Noradrenergic and serotoninergic fibers innervate complementary layers in monkey primary visual cortex: an immunohistochemical study. Proc Natl Acad Sci USA 79: 2401–2405
Parnavelas JG, Moises HC, Speciale SG (1985) The monoaminergic innervation of the rat visual cortex. Proc R Soc Lond B 223: 319–329
Peters A, Kaiserman-Abramof IR (1970) The small pyramidal neuron of the rat cerebral cortex: the perikaryon, dendrites and spines. Am J Anat 127: 321–356
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17: 208–212
Sloper JJ, Hiorns RW, Powell TPS (1979) A qualitative and quantitative electron microscope study of the neurons in the primate motor and somatic sensory cortices. Philos Trans R Soc London Ser B 285: 141–171
Tork I, Hornung J-P, Mulligan KA, van der Loos H (1986) Synaptic connections of serotonergic axons in the molecular layer of the cat's neocortex. Neurosci Lett Suppl 26: S104
Tork I, Mulligan KA (1984) Dense serotoninergic innervation of select cortical neurons in cat neocortex. Soc Neurosci Abstr 10: 63
Watson JL (1958) Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol 4: 475–478
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DeFelipe, J., Jones, E.G. A light and electron microscopic study of serotonin-immunoreactive fibers and terminals in the monkey sensory-motor cortex. Exp Brain Res 71, 171–182 (1988). https://doi.org/10.1007/BF00247532
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00247532