Summary
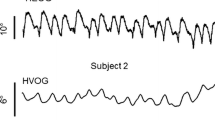
1)Horizontal optokinetic eye nystagmus (OKN) and afternystagmus (OKAN) were recorded in the alert cat (head restrained) in response to velocity steps and sinusoidal optokinetic stimuli. 2)A strong dependency of OKN performance on stimulus pattern was found: responses were most regular and gain was high over a large range of stimulus velocities when the stimulus consisted of a high-contrast random dot pattern. 3) Following velocity steps, OKN showed a small amplitude fast rise in slow phase velocity (SPV) which was followed by a slow build-up to steady state. The amplitude of the initial jump in SPV increased with stimulus amplitude up to 30°/s and saturated afterwards. The plateau level of initial SPV ranged from 5 to 15°/s. 4) The slow build-up of SPV showed non-linearities, i.e. the time to steady state increased with stimulus amplitude and the slow rise of SPV was irregular. In most animals steady state SPV showed no signs of response saturation for step amplitudes up to 60–80°/s or more. The open-loop gain (steady state SPV/ retinal slip velocity) dependend on retinal slip velocity and decreased from 46 at 0.5°/s to 0.4 at about 60°/s. 5) OKAN I and II were consistently observed and occasionally OKAN III was noted. OKAN I durations (mean 13.8 +- 5.1 s) and OKAN II amplitudes were independent of stimulus magnitude. Initial SPV of OKAN I was typically the same as that of OKN, i.e. no fast fall was observed. Cessation of pattern rotation in light, however, produced a fast initial decay of SPV. 6) A least square fitting of OKAN time course was performed with various time functions. The SPV of OKAN I and II was best fitted with a damped sine wave, indicating that cat optokinetic system behaves like a second order underdamped system. 7) Sinusoidal stimuli produced strong response non-linearities. At a given frequency gain decreased with increasing stimulus amplitudes. Gain correlated best with stimulus acceleration. In addition, strong stimuli produced characteristic response distortions. 8) In the visual-vestibular conflict situation vectorial summation of VOR and OKN was observed only with small stimuli.
Similar content being viewed by others
References
Barnes GR, Edge A (1983) Non-linear effects in visual suppression of vestibular nystagmus. Exp Brain Res 52: 9–19
Bond HW, Ho P (1970) Solid miniature silver-silverchloride electrodes for chronic implantations. Electroencephalogr Clin. Neurophysiol 28: 206–208
Brandt Th, Dichgans J, Büchele W (1974) Motion habituation: Inverted self-motion perception and optokinetic afternystagmus. Exp Brain Res 21: 337–352
Buizza A, Schmid R (1982) Visual-vestibular interaction in the control of eye movement: mathematical modelling and computer simulation. Biol Cybern 43: 209–223
Büttner U, Meienberg O, Schimmelpfenning B (1982) The role of he fovea and parafoveal regions in the control of “fast” optokinetic responses in the monkey. In: Roucoux A, Crommelinck M (eds) Physiological and pathological aspects of eye movements. W. Junk Publishers, The Hague, Boston, London, pp 173–179
Cheng M, Outerbridge JS (1975) Optokinetic nystagmus during selective retinal stimulation. Exp Brain Res 23: 129–139
Cohen B, Uemura T, Takemori S (1973) Effects of labyrinthectomy on optokinetic afternystagmus. Equilibrium Res 3: 88–93
Cohen B, Matsuo V, Raphan Th (1977) Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic afternystagmus. J Physiol (Lond) 270: 321–344
Collewijn H (1969) Optokinetic eye movements in the rabbit: input-output relations. Vision Res 9: 117–132
Collewijn H (1972) An analog model of the rabbit's optokinetic system. Brain Res 36: 71–88
Collewijn H (1976) Impairment of optokinetic (after-)nystagmus by labyrinthectomy in the rabbit. Exp Neurol 52: 146–156
Collewijn H, Winterson BJ, van der Steen J (1980) Post-rotatory nystagmus and optokinetic after-nystagmus in the rabbit linear rather than exponential decay. Exp Brain Res 40: 330–338
Collewijn H (1981) The oculomotor system of the rabbit and its plasticity. Springer, Berlin, Heidelberg, New York
Collins WE, Schroeder DJ, Rice N, Mertens RA, Kranz G (1970) Some characteristics of optokinetic eye-movement patterns: A comparative study. Aerospace Med 41: 1251–1262
Demer JL (1981) The variable gain element of the vestibulo-ocular reflex is common to the optokinetic system of the cat. Brain Res 229: 1–13
Dieringer N, Precht W (1982) Compensatory head and eye movements in the frog and their contribution to stabilization of gaze. Exp Brain Res 47: 394–406
Donaghy M (1980) The contrast sensitivity, spatial resolution and velocity tuning of the cat's optokinetic reflex. J Physiol (Lond) 300: 353–365
Dubois MF, Collewijn H (1979a) The optokinetic reactions of the rabbit: relation to the visual streak. Vision Res 19: 9–17
Dubois MF, Collewijn H (1979b) Optokinetic reactions in man elicited by localized retinal motion stimuli. Vision Res 19: 1105–1115
Erickson RG, Barmack NH (1980) A comparsion of the horizontal and vertical optokinetic reflexes of the rabbit. Exp Brain Res 40: 448–456
Evinger C, Fuchs AF (1978) Saccadic, smooth pursuit and optokinetic eye movements of the trained cat. J Physiol (Lond) 285: 209–229
Gioanni H, Rey J, Villalobos J, Bouyer JJ, Gioanni Y (1981) Optokinetic nystagmus in the pigeon (Columba livia). I. Study in monocular and binocular vision. Exp Brain Res 44: 362–370
Godaux E, Gobert C, Halleux J (1983) Vestibuloocular reflex, optokinetic response and their interactions in the alert cat. Exp Neurol 80: 42–54
Haddad GM, Demer JL, Robinson DA (1980) Effect of lesions of the dorsal cap of the inferior olive on the vestibulo-ocular and optokinetic systems of the cat. Brain Res 185: 265–275
Harris LR, Cynader M (1981) The eye movements of the darkreared cat. Exp Brain Res 44: 41–56
Hoffmann K-P, Schoppmann A (1981) A quantitative analysis of the direction-specific response of neurons in the cat's nucleus of the optic tract. Exp Brain Res 42: 146–157
Honrubia V, Scott BJ, Ward PH (1967) Experimental studies on optokinetic nystagmus. I. Normal cats. Acta Oto-Laryngol 64: 388–402
Igarashi M, Takahashi M, Homick JL (1977) Optokinetic nystagmus and vestibular stimulation in squirrel monkey model. Arch Oto-Rhino-Laryngol 218: 115–121
Keller EL, Precht W (1979) Visual-vestibular responses in vestibular nuclear neurons in intact and cerebellectomized, alert cat. Neuroscience 4: 1599–1613
Keller EL, Precht W (1981) Adaptive modification in brainstem pathways during vestibulo-ocular reflex recalibration. In: Flohr H, Precht W (eds) Lesion-induced neuronal plasticity in sensorimotor systems. Springer, Berlin, Heidelberg, pp 284–294
Kennedy H, Courjon JH, Flandrin JM (1982) Vestibulo-ocular reflex and optokinetic nystagmus in adult cats reared in stroboscopic illumination. Exp Brain Res 48: 279–287
Koenig E, Dichgans J (1981) Aftereffects of vestibular and optokinetic stimulation and their interaction. Ann NY Acad Sci 374: 434–445
Komatsuzaki A, Harris HE, Alpert J, Cohen B (1969) Horizontal nystagmus of rhesus monkeys. Acta Oto-laryngologica 67: 535–551
Lisberger SG, Evinger C, Johanson GW, Fuchs AF (1981a) Relationship between eye acceleration and retinal image velocity during foveal smooth pursuit in man and monkey. J Neurophysiol 46: 229–249
Lisberger SG, Miles FA, Optican LM, Eighmy BB (1981b) Optokinetic responses in monkey: underlying mechanisms and their sensitivity to long-term adaptive changes in vestibuloocular reflex. J Neurophysiol 45: 869–890
Maioli C, Precht W, Ried S (1983) Short- and long-term modifications of vestibulo-ocular response dynamics following unilateral vestibular nerve lesions in the cat. Exp Brain Res 50: 259–274
Malcolm R, B.Sc, M.Sc, Melvill Jones G (1970) A quantitative study of vestibular adaptation in humans. Acta otolaryngol 70: 126–135
Oyster CW, Takahashi E, Collewijn H (1972) Direction-selective retinal ganglion cells and control of optokinetic nystagmus in the rabbit. Vision Res 12: 183–193
Paige GD (1983) Vestibuloocular reflex and its interactions with visual following mechanisms in the squirrel monkey. I. Response characteristics in normal animals. J Neurophysiol 49: 134–151
Robinson DA (1977) Linear addition of optokinetic and vestibular signals in the vestibular nucleus. Exp Brain Res 30: 447–450
Robinson DA (1977) Linear addition of optokinetic and vestibular signals in the vestibular nucleus. Exp Brain Res 30: 447–450
Robinson DA (1980) In: Henn V, Cohen B, Young LR (eds),Visual-vestibular interaction in motion perception and the generation of nystagmus. Neurosciences Research Program Bull 18: Nr. 4
Ter Braak JWG (1936) Untersuchungen über optokinetischen Nystagmus. Arch Néerl Physiol 21: 309–376
Van Die G, Collowijn H (1982) Optokinetic nystagmus in man. Role of central and peripheral retina and occurrence of asymmetries. Human Neurobiol 1: 111–119
Waespe W, Huber Th, Henn V (1978) Dynamic changes of optokinetic after-nystagmus (OKAN) caused by brief visual fixation periods in monkeys and in man. Arch Psychiat Nervenkr 226: 1–10
Waespe W, Henn V (1979) The velocity response of vestibular nucleus neurons during vestibular, visual and combined angular acceleration. Exp Brain Res 37: 337–347
Waespe W, Cohen B, Raphan T (1983) Role of the flocculus and paraflocculus in optokinetic nystagmus and visual-vestibular interactions: effects of lesions. Exp Brain Res 50: 9–33
Young LR, Oman CM (1969) Model for vestibular adaptation to horizontal rotation. Aerospace Med 40: 1076–1080
Zee DS, Yee RD, Robinson DA (1976) Optokinetic responses in labyrinthine-defective human beings. Brain Res 113: 423–428
Zee DS, Yamazaki A, Butler PH, Gücer G (1981) Effects of ablation of flocculus and paraflocculus on eye movements in primate. J Neurophysiol 46: 878–899
Zee DS, Butler PH, Optican LM, Tusa RJ, Gücer G (1982) Effects of bilateral occipital lobectomies on eye movements in monkeys: Preliminary observations. In: Roucoux A, Crommelinck M (eds) Physiological and pathological aspects of eye movements. W. Junk Publishers, The Hague, Boston, London, pp 225–232
Author information
Authors and Affiliations
Additional information
Supported by grants nos. 3.505.79 and 3.403.83 from the Swiss National Science Foundation and Dr. Erik Slack-Gyr Foundation
Rights and permissions
About this article
Cite this article
Maioli, C., Precht, W. The horizontal optokinetic nystagmus in the cat. Exp Brain Res 55, 494–506 (1984). https://doi.org/10.1007/BF00235280
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00235280