Abstract
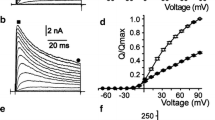
Effects of stilbene disulfonates on single KATP channel currents were investigated in inside-out and outside-out membrane patches from guinea pig ventricular myocytes. All drugs tested, 4,4′-diisothiocyanatostilbene, 2,2′-disulfonic acid (DIDS), 4-acetamido0-4′-isothiocyanatostilbene-2,2′-disulfonic acid (SITS), 4,4′-dinitrostilbene-2,2′-disulfonic acid (DNDS), and 4,4′-diaminostilbene-2,2′-disulfonic acid (DADS), inhibited the KATP channel when they were applied to the intracellular, but not extracellular side of the membrane patch. Inhibitory actions of DIDS and SITS were irreversible, whereas those induced by DNDS and DADS were reversible. KATP channel inhibition was concentration dependent with an order of potency of DIDS>SITS ≈ DNDS > DADS; the Hill coefficient was close to unity for each drug. No change in channel conductance was observed during exposure to DIDS or DNDS; however, channel kinetics was altered. Distribution of the open time within bursts and that between bursts could be described by a single exponential relation in the absence and presence of DIDS or DNDS. The time constant of the open time within bursts was not altered, but that between bursts was decreased by DIDS (from 40.0±8.1 to 29.8±6.7 msec, P< 0.05) and by DNDS (from 43.1±9.3 to 31.9±7.1 msec, P<0.05). Distributions of closed time within bursts were also fitted to a single exponential function both in the absence and presence of drugs, while those of the closed time between bursts were fitted to a single exponential function in the absence of drugs, but a double exponential function was required in the presence of drugs. The rates of onset and development of channel inhibition by DIDS and DNDS appeared to be concentration dependent; a longer time was required to reach a new steady-state of channel activity as drug concentration was decreased. Inhibition by DIDS or DNDS was regulated by intracellular pH; inhibition was greater during acidic conditions. For DIDS (0.1 mm), the open probability (P o) expressed as a fraction of the value before drug application was 42.9±8.3% at pH 7.4 and 8.2±6.6% at pH 6.5 (P<0.01); corresponding values for DNDS (1 mm) were 39.6±17.6 and 8.9 ±5.8%, respectively (P<0.01). From these data, we conclude that stilbene disulfonates block the KATP channel by binding to their target site with one-to-one stoichiometry. Similar to glibenclamide, the binding of stilbene disulfonates may reflect interpolation in an “intermediate lipid compartment” between the cytosolic drug and the site of drug action.
Similar content being viewed by others
References
Anderson, M.P., Berger, H.A., Rich, D.P., Gregory, R.J., Smith, A.E., Welsh, M.J. 1991. Nucleotide triphosphates are required to open the CFTR chloride channel. Cell 67:775–784
Ashcroft, F.M. 1988. Adenosine 5′-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci. 11:97–118
Ashford, M.L.J., Sturgess, N.C., Trout, N.J., Gardner, N.J., Hales, C.N. 1988. Adenosine-5′-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pfluegers Arch. 412:297–304
Bhattahcarya, C.G. 1967. A simple method of resolution of a distribution into Gaussian components. Biometrics 23: 115–135
Cabantchik, Z.I., Knauf, P.A., Rothstein, A. 1978. The anion transport system of the red blood cell: The role of membrane protein evaluated by the use of ‘probes’. Biochim. Biophys. Acta 515:239–302
Cook, D.L., Hales, C.N. 1984. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311:271–311
Cook, N.S. 1988. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol. Sci. 9:21–28
Edwards, G., Weston, A.H. 1990. Potassium channel openers and vascular smooth muscle relaxation. Pharmacol. Ther. 48:237–258
Fabiato, A., Fabiato, F. 1979. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. (Paris) 75:463–505
Fan, Z., Nakayama, K., Hiraoka, M. 1990. Pinacidil activates the ATP-sensitive K+ channel in inside-out and cell-attached patch membranes of guinea-pig ventricular myocytes. Pfluegers Arch. 415:387–394
Fan, Z., Nakayama, K., Sawanobori, T., Hiraoka, M. 1992. Aromatic aldehydes and ketones open ATP-sensitive K+ channels in guinea-pig ventricular myocytes. Pfluegers Arch. 421:409–415
Findlay, I. 1992a. Inhibition of ATP-sensitive K+ channels in cardiac muscle by the sulphonylurea drug glibenclamide. J. Pharmacol. Exp. Ther. 261:540–545
Findlay, I. 1992b. Effects of pH upon the inhibition by sulphonylurea drugs of ATP-sensitive K+ channels in cardiac muscle. J. Pharmacol. Exp. Ther. 262:71–79
Fröhlich, O. 1982. The external anion binding site of the human erythrocyte anion transporter: DNDS binding and competition with chloride. J. Membrane Biol. 65:111–123
Furukawa, T., Fan, Z., Sawanobori, T., Hiraoka, M. 1993. Modification of the adenosine 5′-triphosphate-sensitive K+ channel by trypsin in guinea-pig ventricular myocytes. J. Physiol. 466:707–726
Groschner, K., Kukovetz, W.R. 1992. Voltage-sensitive chloride channels of large conductance in the membrane of pig aortic endothelial cells. Pfluegers Arch. 421:209–217
Ho, K., Nichols, C.G., Lederer, W.J., Lytton, J., Vassilev, P.M., Kanazirska, M.V., Hebert, S.C. 1993. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362:31–38
Kakei, M., Noma, A. 1984. Adenosine 5′-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. J. Physiol. 352:265–284
Kakei, M., Noma, A., Shibasaki, T. 1985. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J. Physiol. 363:441–462
Kantor, P.F., Coetzee, W.A., Carmeliet, E.E., Dennis, S.C., Opie, L.H. 1990. Reduction of ischemic K+ loss and arrhythmias in rat hearts: Effect of glibenclamide, a sulfonylurea. Circ. Res. 66:478–485
Kokubun, S., Saigusa, A., Tamura, T. 1991. Blockade of Cl channels by organic and inorganic blockers in vascular smooth muscle cells. Pfluegers Arch. 418:204–213
Maddy, A.H. 1964. A fluorescent label for the outer components of the plasma membrane. Biochim. Biophys. Acta 88:390–399
Nelder, J.A., Mead, R. 1965. A simplex method for function minimization. Comput. J. 7:308–313
Noma, A. 1983. ATP-regulated K+ channels in cardiac muscle. Nature 305:147–148
Rorsman, P., Trube, G. 1985. Glucose dependent K+ channels in pancreatic β-cells are regulated by intracellular ATP. Pfluegers Arch. 405:305–309
Sheppard, D.N., Welsh, M.J. 1992. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J. Gen. Physiol. 100:573–591
Spruce, A.E., Standen, N.B., Stanfield, P.R. 1985. Voltage-dependent, ATP-sensitive potassium channels of skeletal muscle membrane. Nature 316:736–738
Spruce, A.E., Standen, N.B., Stanfield, P.R. 1987. Studies on the unitary properties of adenosine-5′-triphosphate-regulated potassium channels of frog skeletal muscle. J. Physiol. 382:213–237
Standen, N.B., Quayle, J.M., Davies, N.W., Brayden, J.E., Huang, Y., Nelson, M.T. 1989. Hyperpolarizing vasodilators activate ATP-sensitive K channels in arterial smooth muscle. Science 245:177–180
Trube, G., Hescheler, J. 1984. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pfluegers Arch. 401:178–184
Wang, W., Schwab, A., Giebisch, G. 1990. Regulation of smallconductance K+ channel in apical membrane of rat cortical collecting tubule. Am. J. Physiol. 259:F494-F502
Woll, K.H., Leibowitz, M.D., Neumcke, B., Hille, B. 1987. A high-conductance anion channel in adult amphibian skeletal muscle. Pfluegers Arch. 410:632–640
Wolleben, C.D., Sanguinetti, M.C., Siegel, P.K.S. 1989. Influence of ATP-sensitive potassium modulators on ischemiainduced fibrillation in isolated rat hearts. J. Mol. Cell. Cardiol. 21:783–788
Author information
Authors and Affiliations
Additional information
The authors wish to thank Dr. Z. Fan for a helpful discussion during the course of experiments, Dr. A.L. Bassett for his reading and commenting on the manuscript, Ms. N. Fujita for her skillful secretarial aid, and Mrs. Y. Sugimoto for her technical assistance. This work is supported by Grant-in-aid for scientific research. from the Ministry of Education, Science and Culture of Japan, and by a Grant-in-aid from the Japanese Research Promotion Society for C ardiovascular Deseases. T.F. is a current recipient of Fellowships of the Japan Society for the Promotion of Science for Japanese Junior Scientists.
Rights and permissions
About this article
Cite this article
Furukawa, T., Virág, L., Sawanobori, T. et al. Stilbene disulfonates block ATP-sensitive K+ channels in guinea pig ventricular myocytes. J. Membarin Biol. 136, 289–302 (1993). https://doi.org/10.1007/BF00233668
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00233668