Abstract
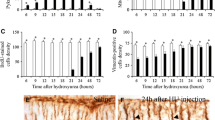
Sprague-Dawley rats received a single dose of 2 Gy X-rays at the age of 1 or 3 days and were killed at different intervals. Dying cells with the morphological characteristics of apoptosis appeared in the external and internal granular layers (EGL and IGL) and white matter (WM) of the cerebellum, mainly 3–6 h after irradiation, and decreased thereafter to reach normal values between 48 h and 5 days later. This process was curbed by the injection of cycloheximide at a dose of 1 μg/g body weight. In addition, the number of mitoses in EGL rapidly decreased after irradiation and did not reach normal values until a few days later. Proliferating cell nuclear antigen (PCNA)-immunoreactive cells, which were chiefly found in EGL but also in IGL and WM, dramatically decreased in number from 3 to 48 h after irradiation. PCNA-immunoreactive cells reappeared and reached age-matched values in the following days. Hu (considered as an early neuronal marker) and vimentin immunocytochemistry disclosed that Hu-nonreactive cells in the upper level of EGL, Hu-immunoreactive cells in the inner level of EGL, Bergmann glia and many astrocytes in WM, as well as many non-typified cells in WM, were radiosensitive populations, whereas Purkinje cells were not. The present results indicate that irradiation at P1 or P3 blocks mitosis in EGL and kills sensitive cells mainly in the late G1 and S phases of the cell cycle, probably by apoptosis through a protein synthesis-mediated process. Radiosensitive cells are germinal cells and neuroblasts in EGL, Bergmann glia, astrocytes in WM, and non-typified cells, probably glial cell precursors, in WM. Surviving cells in EGL and PCNA-immunoreactive cells in other cortical layers and white matter reconstitute the cerebellum following a single dose of X-rays.
Similar content being viewed by others
References
Altman J, Anderson WJ (1972) Experimental reorganization of the cerebellar cortex. I. Morphological effects of elimination of all microneurons with prolonged x-irradiation started at birth. J Comp Neurol 146:355–406
Altman J, Anderson WJ, Wright KA (1969) Reconstitution of the external granular layer of the cerebellar cortex in infant rats after low-level X-irradiation. Anat Rec 163:453–472
Anderson NE, Roseblum MK, Graus F, Wiley RG, Posner JB (1988) Autoantibodies in paraneoplastic syndromes associated with small-cell lung cancer. Neurology 38:1391–1398
Bravo R (1986) Synthesis of the nuclear protein cyclin (PCNA) and its relationship with DNA replication. Exp Cell Res 163:287–293
Bravo R, Celis JE (1980) A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol 84:795–802
Bravo R, Macdonald-Bravo H (1984) Induction of the nuclear protein cyclin in quiescent mouse 3T3 cells stimulated by serum and growth factors: correlation with DNA synthesis. EMBO J 3:3177–3181
Bravo R, Macdonald-Bravo H (1987) Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol 105:1549–1554
Bravo R, Frank R, Blundell PA, Macdonald-Bravo H (1987) Cyclin/PCNA is the auxiliary protein of DNA polymerase δ. Nature 326:515–517
Celis JE, Madsen P, Celis A, Nielsen HV, Gesser B (1987) Cyclin (PCNA, auxiliary protein of DNA polymerase δ) is a central component of the pathway(s) leading to DNA replication and cell division. FEBS Lett 220:1–7
Dalmau J, Furneaux HM, Gralla RJ, Kris MG, Posner JB (1990) Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer: a quantitative Western blot analysis. Ann Neurol 27:544–552
Dalmau J, Graus F, Rosenblum MK, Posner JB (1992) Anti-Hu-associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine 71:59–72
Das GD (1977) Experimental analysis of embryogenesis of cerebellum in rat. II. Morphogenetic malformations following X-ray irradiation on day 18 of gestation. J Comp Neurol 176:435–452
Ferrer I (1992) The effect of cycloheximide on natural and X-ray-induced cell death in the developing cerebral cortex. Brain Res 588:351–357
Ferrer I, Serrano t, Alcántara S, Tortosa A, Graus F (1993) X-ray-induced cell death in the developing hippocampal cortex involves neurons and requires protein synthesis. J Neuropathol Exp Neurol 52:370–378
Furneaux HM, Rosenblum MK, Dalmau J, Wong E, Woodruff P, Graus F, Posner JB (1990) Selective expression of Purkinje cell antigens in tumor tissue from patients with paraneoplastic cerebellar degeneration. N Engl J Med 322:1844–1851
Gonzalez-Martín C, de Diego I, Crespo D, Fairén A (1992) Transient c-fos expression accompanies naturally occurring cell death in the developing interhemispheric cortex of the rat. Dev Brain Res 68:83–95
Graus F, Ferrer I (1990) Analysis of a neuronal antigen (Hu) expression in the developing rat brain detected by autoantibodies from patients with paraneoplastic encephalomyelitis. Neurosci Lett 112:14–18
Graus F, Cordon-Cardo C, Posner J (1985) Neuronal antinuclear antibody in sensory neuronopathy from lung cancer. Neurology 35:538–543
Graus F, Elkon KB, Cordon-Cardo C, Posner JB (1986) Sensory neuronopathy and small cell lung cancer. Antineuronal antibody that also reacts with the tumor. Am J Med 80:45–52
Harmon BV, Allan DJ (1988) X-ray-induced cell death by apoptosis in the immature rat cerebellum. Scanning Microsc 2:561–568
Hicks SP (1958) Radiation as an experimental tool in mammalian developmental neurology. Physiol Rev 38:337–356
Hicks SP, D'Amato CJ (1966) Effects of ionizing radiation on mammalian development. In: Woollam DHM (ed) Advances in teratology. Logos Press, London, pp 196–250
Hicks SP, D'Amato CJ, Coy MA, O'Brien ED, Thruston JM, Foftes DL (1961) Migrating cells in the developing nervous system studied by their radiosensitivity and tritiated thymidine uptake. In: Fundamental aspects of Radiosensitivity; Brookhaven Symposium Biology no. 14. Upton, New York, pp 246–261
Inouye M (1979) Cerebellar malformations in prenatally X-irradiated rats: quantitative analysis and detailed description. Teratology 20:353–364
Inouye M, Kameyama Y (1983) Cell death in the developing rat cerebellum following X-irradiation of 3 to 100 rad: a quantitative study. J Radiat Res (Tokyo) 24:259–269
Inouye M, Tamaru M, Kameyama Y (1992) Effects of cycloheximide and actinomycin D on radiation-induced apoptotic cell death in the developing mouse cerebellum. Int J Rad Biol 61:669–674
Kurki P, Ogata K, Tan EM (1988) Monoclonal antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as probes for proliferating cells by immunofluorescence microscopy and flow cytometry. J Immunol Methods 109:49–59
Marusich MF, Weston JA (1992) Identification of early neurogenic cells in the neural cell lineage. Dev Biol 149:295–306
Matthews MB, Bernstein RM, Franza BR, Garrels JI (1984) Identity of the proliferating cell nuclear antigen and cyclin. Nature 309:374–376
Ogata K, Kurki P, Celis JE, Nakamura RM, Tan EM (1987) Monoclonal antibodies to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res 168:475–486
Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, Posner JB, Furneaux HM (1991) HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell 67:325–333
Takasaki J, Deu JS, Tan EM (1981) A nuclear antigen associated with cell proliferation and blast transformation. Its distribution in synchronized cells. J Exp Med 156:1899–1909
Takasaki Y, Fishwild D, Tan EM (1984) Characterization of proliferating cell nuclear antigen recognized by autoantibodies in lupus sera. J Exp Med 159:981–992
Author information
Authors and Affiliations
Additional information
This work was supported in part by CEC project FI3P-CT92-0015 and FIS grant 93-131. M. Olivé is the recipient of a grant from the Pi i Sunyer Foundation. R. Rivera has a grant from the CIRIT
Rights and permissions
About this article
Cite this article
Ferrer, I., Serrano, T., Rivera, R. et al. Radiosensitive populations and recovery in X-ray-induced apoptosis in the developing cerebellum. Acta Neuropathol 86, 491–500 (1993). https://doi.org/10.1007/BF00228585
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228585