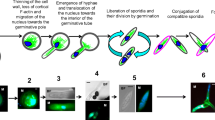
Summary
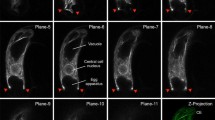
F-actin distribution during male meiosis in Magnolia soulangeana was studied by means of fluorescence microscopy following staining with rhodaminephalloidin. Actin filaments were observed to persist during all of the developmental stages of meiosis. Four main types of configurations were recognized: (1) peripheral filaments underlying the plasma membrane (cortical network); (2) filaments dispersed throughout the inner cytoplasm (central cytoplasmic network); (3) filaments associated with the meiotic spindles; (4) filaments associated with the phragmoplasts. The cortical and central cytoplasmic filaments exhibited different behaviours. Whereas the cortical network remained present in an apparently unchanged form during all of the meiotic stages, the central cytoplasmic filaments, although they never completely disappeared, were reduced and concentrated around the nucleus at the end of prophase. At metaphase, fluorescent spindles consisting of filament bundles running from pole to pole or being interrupted at the equatorial zone could be seen. At the end of both the first and second division of meiosis, fluorescent bands of filaments (disks) appeared at the level of the cell division planes (equatorial regions) where cleavage furrows were constituted. These cleavage furrows did not form when floral buds were “cultivated” in a cytochalasin-containing medium. Our results show that during microsporogenesis in M. soulangeana the actin filaments constitute a highly complex and dynamic system that is involved in particular in cytoplasm cleavage of the meiocytes.
Similar content being viewed by others
References
Bednara J, Willemse MTM, Van Lammeren AAM (1990) Organization of the actin cytoskeleton during megasporogenesis in Gasteria verrucosa visualized with fluorescent-labelled phalloidin. Acta Bot Neerl 39:43–48
Brown RC, Lemmon BE (1989) Minispindles and cytoplasmic domains in microsporogenesis of orchids. Protoplasma 148:26–32
Brown RC, Lemmon BE (1991) Pollen development in orchids. 1. Cytoskeleton and the control of division plane in irregular patterns of cytokinesis. Protoplasma 163:9–18
Clayton L, Lloyd CW (1985) Actin organization during the cell cycle in meristematic plant cells. Exp Cell Res 156:231–238
Derksen J, Traas JA, Oostendorp T (1986) Distribution of actin filaments in differentiating cells of Equisetum hyemale root tips. Plant Sci 43:77–81
Derksen J, Wilms FHA, Pierson ES (1990) The plant cytoskeleton: its significance in plant development. Acta Bot Neerl 39:1–18
Dinis AM, Mesquita JF (1990a) Ultrastructure of microsporogenesis in Magnolia soulangeana Soul. (Magnoliaceae). V. The cytokinesis in the meiocytes. In: XXV Meet Port Soc Electron Microsc. S.P.M.E., Porto, p 61 (abstr)
Dinis AM, Mesquita JF (1990b) Ultrastructure of microsporogenesis in Magnolia soulangeana Soul. (Magnoliaceae). IV. Ultrastructural and cytochemical aspects of the cytoplasmic evolution in the meiocytes. In: XXV Meet Port Soc Electron Microsc. S.P.M.E., Porto, p 59 (abstr)
Gabara B (1971) Cytokinesis in pollen mother cells. II. Magnolia soulangeana Soul. Biochem Physiol Pflanz 162:450–458
Goto Y, Ueda K (1988) Microfilament bundles of F-actin in Spirogyra observed by fluorescence microscopy. Planta 173:442–446
Gunning BES, Wick S (1985) Preprophase band, phragmoplasts and spatial control of cytokinesis. J Cell Sci 2:157–179
Hogan CJ (1987) Microtubule patterns during meiosis in two higher plant species. Protoplasma 138:126–136
Lloyd CW (1988) Actin in plants. J Cell Sci 90:185–188
Lloyd CW, Traas JA (1988) The role of F-actin in determining the division plane of carrot suspension cells. Drug studies. Development 102:211–221
Mangeat P, Burridge K (1984) Actin-membrane interaction in fibroblasts: what proteins are involved in this association? J Cell Biol 99:95s-103s
Palevitz BA (1980) Comparative effects of phalloidin and cytochalasin B on motility and morphogenesis in Allium. Can J Bot 58:773–784
Parthasarathy MV, Perdue TD, Witztum A, Alvernaz J (1985) Actin network as a normal component of the cytoskeleton in many vascular plant cells. Am J Bot 72:1318–1323
Pickett-Heaps JD (1975) Green algae. Structure, reproduction and evolution in selected genera. Sinauer Associates, Sunderland, Mass.
Pierson ES (1988) Rhodamine-palloidin staining of F-actin in pollen after dimethylsulphoxide permeabilization. A comparison with the conventional formaldehyde preparation. Sex Plant Reprod 1:83–87
Schliwa M (1981) Proteins associated with cytoplasmic actin. Cell 25:587–590
Schmit AC, Lambert AM (1987) Characterization and dynamics of cytoplasmic F-actin in higher plant endosperm cells during interphase, mitosis, and cytokinesis. J Cell Biol 105:2157–2166
Schmit AC, Lambert AM (1988) Plant actin filament and microtubule interactions during anaphase-telophase transition: effects of antagonist drugs. Biol Cell 64:309–319
Schmit AC, Lambert AM (1990) Microinjected fluorescent phalloidin in vivo reveals the F-actin dynamics and assembly in higher plant mitotic cells. Plant Cell 2:129–138
Schroeder TE (1976) Actin in dividing cells: evidence for its role in cleavage but not mitosis. In: Goldman R, Polland T, Rosen-baum J (eds) Cell Motility (Book A). Cold Spring Harbor Conf, pp 265–268
Seagull RW, Falconer MM, Weerdenburg CA (1987) Microfilaments: dynamic arrays in higher plant cells. J Cell Biol 104:995–1004
Sheldon JM, Hawes C (1988) The actin cytoskeleton during male meiosis in Lilium. Cell Biol Int Rep 12:471–476
Staiger CJ, Cande WZ (1991) Microfilament distribution in maize meiotic mutants correlates with microtubule organization. Plant Cell 3:637–644
Staiger CJ, Schliwa M (1987) Actin localization and function in higher plants. Protoplasma 141:1–12
Tanaka I (1991) Microtubule-determined plastid distribution during microsporogenesis in Lilium longiflorum. J Cell Sci 99:21–31
Tewinkel M, Kruse S, Quader H, Volkmann D, Sievers A (1989) Visualization of actin filament pattern in plant cells without pre-fixation. A comparison of differently modified phallotoxins. Protoplasma 149:178–182
Traas JA, Doonan JH, Rawlins DJ, Shaw PJ, Watts J, Lloyd CW (1987) An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J Cell Biol 105:387–395
Traas JA, Burgain S, Dumas de Vaulx R (1989) The organization of the cytoskeleton during meiosis in eggplant [Solanum melongena (L.)]: microtubules and F-actin are both necessary for coordinated meiotic division. J Cell Sci 92:541–550
Van Lammeren AAM, Keijzer CJ, Willemse MTM, Kieft H (1985) Structure and function of the microtubular cytoskeleton during pollen development in Gasteria verrucosa (Mill.) H. Duval. Planta 165:1–11
Van Lammeren AAM, Bednara J, Willemse MTM (1989) Organization of the actin cytoskeleton during pollen development in Gasteria verrucosa (Mill.) H. Duval visualized with rhodamine-phalloidin. Planta 178:531–539
Wick SM (1991) Spatial aspects of cytokinesis in plant cells. Curr Opinion Cell Biol 3:253–260
Wieland T (1977) Modification of actins by phallotoxins. Naturwissenschaften 64:303–309
Wulf E, Deboden A, Bautz FA, Faulstich H, Wieland T (1979) Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci USA 76:4498–4502
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dinis, A.M., Mesquita, J.F. The F-actin distribution during microsporogenesis in Magnolia soulangeana Soul. (Magnoliaceae). Sexual Plant Reprod 6, 57–63 (1993). https://doi.org/10.1007/BF00227584
Issue Date:
DOI: https://doi.org/10.1007/BF00227584