Summary
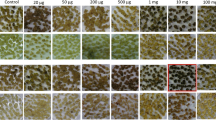
Stable transformed Black Mexican Sweet (BMS) maize callus was recovered from suspension culture cells bombarded with plasmid DNA that conferred resistance to the herbicide bialaphos. Suspension culture cells were bombarded with a mixture of two plasmids. One plasmid contained a selectable marker gene, bar, which encoded phosphinothricin acetyl transferase (PAT), and the other plasmid encoded a screenable marker for β-glucuronidase (GUS). Bombarded cells were selected on medium containing the herbicide bialaphos, which is cleaved in plant cells to yield phosphinothricin (PPT), an inhibitor of glutamine synthetase. The bialaphos-resistant callus contained the bar gene and expressed PAT as assayed by PPT inactivation. Transformants that expressed high levels of PAT grew more rapidly on increasing concentrations of bialaphos than transformants expressing low levels of PAT. Fifty percent of the bialaphos-resistant transformants tested (8 of 16) expressed the nonselected gene encoding GUS.
Similar content being viewed by others
References
Chomet PS, Wessler S, Dellaporta SL (1987) Inactivation of the maize transposable element Activator (Ac) is associated with its DNA modification. EMBO J 6:295–302
De Block M, Botterman J, Vandiwiele M, Dockx J, Thoen C, Gossele V, Rao Movva N, Thompson C, Van Montagu M, Leemans J (1987) Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J 6:2513–2518
De Block M, De Brouwer D, Tenning P (1989) Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of the bar and neo genes in the transgenic plants. Plant Physiol 91:694–701
Dekeyser R, Claes B, Marichal M, Van Montagu M, Caplan A (1989) Evaluation of selectable markers for rice transformation. Plant Physiol 90:217–223
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13
Folger KR, Wong EA, Wahl G, Capecchi MR (1982) Patterns of integration of DNA microinjected into cultured mammalian cells: Evidence of homologous recombination between injected plasmid DNA molecules. Mol Cell Biol 2:1372–1387
Fromm ME, Taylor LP, Walbot V (1986) Stable transformation of maize after gene transfer by electroporation. Nature 319:791–793
Hauptmann RM, Vasil V, Ozias-Aikins P, Tabaeizadeh Z, Rogers SG, Fraley RT, Horsch RB, Vasil IK (1988) Evaluation of selectable markers for obtaining stable transformants in the Gramineae. Plant Physiol 86:602–606
Heyser JW (1984) Callus and shoot regeneration from protoplasts of Proso millet (Panicum miliaceum L.). Z Pflanzenphysiol 113:233–239
Jefferson RA (1987) Assaying chimeric genes in plants:the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Klein TM, Wolf ED, Wu R, Sanford JC (1987) High-velocity microprojectiles for delivering nucleic acids into living cells. Nature 327:70–73
Klein TM, Fromm M, Weissinger A, Tomes D, Schaaf S, Sletten M, Sanford JC (1988a) Transfer of foreign genes into maize cells with high-velocity microprojectiles. Proc Natl Acad Sci USA 85:4305–4309
Klein TM, Gradziel T, Fromm ME, Sanford JC (1988b) Factors influencing gene deliver into Zea mays cells by high-velocity microprojectiles. Bio/Technol 6:559–563
Klein TM, Harper EC, Svab Z, Sanford JC, Fromm ME, Maliga P (1988c) Stable genetic transformation of intact Nicotiana cells by the particle bombardment process. Proc Natl Acad Sci USA 85:8502–8505
Klein TM, Kornstein L, Sanford JC, Fromm ME (1989) Genetic transformation of maize cells by particle bombardment. Plant Physiol 91:440–444
Lörz H, Baker B, Schell J (1985) Gene transfer to cereal cells mediated by protoplast transformation. Mol Gen Genet 199:178–182
Lyznik LA, Ryan RD, Ritchie SW, Hodges TK (1989) Stable co-transformation of maize protoplasts with gusA and neo genes. Plant Mol Biol 13:151–161
McCabe DE, Swain WF, Martinell BJ, Cristou P (1988) Stable transformation of soybean (Glycine max) by particle acceleration. Bio/Technol 6:923–926
Mendel RR, Muller B, Schulze J, Kolesnikov V, Zelenin A (1989) Delivery of foreign genes to intact barley cells by high-velocity microprojectiles. Theor Appl Genet 78:31–34
Mettler IJ (1987) A simple and rapid method for minipreparation of DNA from tissue cultured plant cells. Plant Mol Biol Rep 5:346–349
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nagy F, Morelli G, Fraley RT, Rogers SG, Chua N-H (1985) Photoregulated expression of a pea rbcS gene in leaves of transgenic plants. EMBO J 2:3063–3068
Okada K, Takeve I, Nagata T (1986) Expression and integration of genes introduced into highly synchronized plant protoplasts. Mol Gen Genet 205:398–403
Prioli LM, Sondahl MR (1989) Plant regeneration and recovery of fertile plants from protoplasts of maize (Zea mays L.). Bio/Technol 7:589–594
Rhodes CA, Pierce DA, Mettler IJ, Mascarenhas D, Detmer JJ (1988) Genetically transformed maize plants from protoplasts. Science 240:204–207
Shillito RD, Carswell GK, Johnson CM, DiMaio JJ, Harms CT (1989) Regeneration of fertile plants from protoplasts of elite inbred maize. Bio/Technol 7:581–587
Shimamoto K, Terada R, Izawa T, Fujimoto H (1989) Fertile transgenic rice plants regenerated from transformed protoplasts. Nature 338:274–276
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Thompson CK, Rao Movva N, Tizard R, Crameri R, Davies JE, Lauwereys M, Botterman J (1987) Characterization of the herbicide resistance gene bar from Streptomyces hygroscopicus. EMBO J 6:2519–2623
Wang Y-C, Klein TM, Fromm M, Cao J, Sanford JC, Wu R (1988) Transient expression of foreign genes in rice, wheat, and soybean cells following particle bombardment. Plant Mol Biol 11:433–439
Yang H, Zhang MH, Davey MR, Mulligan BJ, Cocking EC (1988) Production of kanamycin-resistant rice tissues following DNA uptake into protoplasts. Plant Cell Rep 7:421–425
Author information
Authors and Affiliations
Additional information
Cmmunicated by P. Maliga
Rights and permissions
About this article
Cite this article
Spencer, T.M., Gordon-Kamm, W.J., Daines, R.J. et al. Bialaphos selection of stable transformants from maize cell culture. Theoret. Appl. Genetics 79, 625–631 (1990). https://doi.org/10.1007/BF00226875
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00226875