Summary
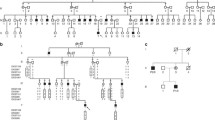
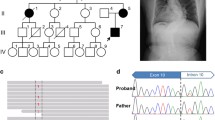
Type I collagen chains of a proband from a family with recurrent lethal osteogenesis imperfecta (OI) migrated as a doublet when submitted to gel electrophoresis. Cyanogen bromide (CNBr) peptide mapping demonstrated that the post-translational over-modifications were initiated in α1ICB7. Chemical cleavage of cDNA-RNA heteroduplexes identified a mismatch in the α1I cDNA; this mismatch was subsequently confirmed by sequencing a 249-bp fragment amplified by the polymerase chain reaction. A G to T transition in the second base of the first codon of exon 41 resulted in the substitution of glycine 802 by valine. This mutation impaired collagen secretion by dermal fibroblasts. The over-modified chains were retained intracellularly and melted at a lower temperature than normal chains. Collagen molecules synthesized by parental fibroblasts had a normal electrophoretic mobility, but hybridization of genomic DNA with allele-specific oligonucleotides revealed the presence of the mutant allele in the mother's leukocytes. The mutation was not detected in her fibroblasts consistent with the protein data. These results support the hypothesis that somatic and germ-line mosaicism in the phenotypically normal mother explain the recurrence of OI.
Similar content being viewed by others
References
Bakker E, Veenema H, Den Dunnen JT, Van Broeckhoven C, Grootscholten PM, Bonten EJ, Van Ommen GJB, Pearson PL (1989) Germinal mosaicism increases the recurrence risk for new Duchenne muscular dystrophy mutations. J Med Genet 26:553–559
Barsh GS, Byers PH (1981) Reduced secretion of structurally abnormal type I procollagen in a form of osteogenesis imperfecta. Proc Natl Acad Sci USA 78:5142–5146
Bernard MP, Chu ML, Myers JC, Ramirez F, Eikenberry EF (1983) Nucleotide sequences of cDNA for the proα1I chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry 22:5213–5223
Bonaventure J, Cohen-Solal L, Lasselin C, Allain JC, Maroteaux P (1986) Abnormal procollagen synthesis in fibroblasts from three patients of the same family with a severe form of osteogenesis imperfecta (type III). Biochim Biophys Acta 889:23–34
Bonaventure J, Zylberberg L, Cohen-Solal L, Allain JC, Maroteaux P (1989) A new lethal brittle bone syndrome with increased amount of type V in a patient. Am J Med Genet 33:299–310
Bruckner P, Prockop DJ (1981) Proteolytic enzymes as probes for the triple helical conformation of collagen. Anal Biochem 110:360–368
Byers PH (1989) Inherited disorders of collagen gene structure and expression. Am J Med Genet 34:72–80
Byers PH (1990) Brittle bones — fragile molecules: disorders of collagen gene structure and expression. Trends Genet 6:293–300
Byers PH, Tsipouras P, Bonadio JF, Starman BJ, Schwartz RC (1988) Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet 42:237–248
Byers PH, Wallis GA, Willing MC (1991) Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet 28:433–442
Cohen-Solal L, Bonaventure J, Maroteaux P (1991) Dominant mutations in familial lethal and severe osteogenesis imperfecta. Hum Genet 87:297–301
Cohn DH, Starman B, Blumberg B, Byers P (1990a) Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). Am J Hum Genet 46:591–601
Cohn DH, Wallis G, Zhang X, Byers P (1990b) Serine for glycine substitutions in the all chain of type I collagen: biological plasticity in the Gly-Pro-Hyp clamp at the carboxyl-terminal end of the triple helical domain. Matrix 10:236
Cole WG, Chan D (1981) Analysis of the heterogeneity of human collagens by two-dimensional polyacrylamide gel electrophoresis. Biochem J 197:377–383
Cole WG, Chow CW, Rogers JG, Bateman JF (1990) The clinical features of three babies with osteogenesis imperfecta resulting from the substitution of glycine by arginine in the proα1I chain of type I procollagen. J Med Genet 27:228–235
Constantinou CD, Pack M, Young SB, Prockop D (1990) Phenotypic heterogenity of osteogenesis imperfecta: the mildly affected mother of a proband with a lethal variant has the same mutation substituting cysteine for α1-glycine 904 in a type I procollagen gene (COL1A1). Am J Hum Genet 47:670–679
Dahl HHM, Lamande SR, Cotton RAH, Bateman JF (1989) Detection and localization of base changes in RNA using a chemical cleavage procedure. Anal Biochem 183:263–268
Hall JG (1988) Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am J Hum Genet 43:355–363
Kuivaniemi H, Tromp G, Prockop D (1991) Mutations is collagen genes: causes of rare and some common diseases in humans. FASEB J 5:2052–2060
Lamande SR, Dahl HHM, Cole WG, Bateman JF (1989) Characterization of point mutations in the collagen COL1A1 and COL1A2 genes causing lethal perinatal osteogenesis imperfecta. J Biol Chem 264:15809–15812
Levinson B, Lehesjoki A-E, Chapelle A de la, Gitschier J (1990) Molecular analysis of hemophilia A mutations in the Finnish population. Am J Hum Genet 46:53–62
Miller EJ, Rhodes RK (1982) Preparation and characterization of the different types of collagen. Methods Enzymol 82A:33–64
Neville DM, Glossmann H (1974) Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol 37B:92–102
Patterson E, Smiley E, Bonadio J (1989) RNA sequence analysis of a perinatal lethal OI mutation. J Biol Chem 264:10083–10087
Prockop DJ (1990) Mutations that alter the primary structure of type I collagen. J Biol Chem 265:15349–15352
Prockop DJ, Constantinou CD, Dombrowski KE, Hojima Y, Kadler KE, Kuivaniemi H, Tromp G, Vogel B (1989) Type I procollagen: the gene-protein system that harbors most of the mutations causing osteogenesis imperfecta and probably more common heritable connective tissue disorders. Am J Med Genet 34:60–67
Ramirez F, Boast S, D'Alessio M, Lee B, Prince J, Su MW, Vissing H, Yoshioka H (1990) Fibrillar collagen genes. Structure and expression in normal and diseased states Ann NY Acad Sci 590:74–80
Soriano P, Jaenisch R (1986) Retroviruses as probes for mammalian development: allocation of cells to the somatic and germ cell lineages. Cell 46:19–29
Stacey A, Bateman JF, Choi T, Mascara T, Cole WG, Jaenisch R (1988) Perinatal lethal OI in transgenic mice bearing an engineered mutant proα1I collagen gene. Nature 332:131–136
Starman BJ, Eyre D, Charbonneau H, Harrylock M, Veis MA, Weiss L, Graham JMJr, Byers PH (1989) Osteogenesis imperfecta: the position of substitution for glycine by cysteine in the triple helical domain of the proα1I chains of type I collagen determines the clinical phenotype. J Clin Invest 84:1206–1214
Stegeman H (1958) Mikrobestimmung von Hydroxyprolin mit Chloramin T und p. Dimethylaminobenzaldehyd. Z Physiol Chem 311:41–45
Sykes B (1990) Bone disease cracks genetics. Nature 348:18–19
Tsuneyoshi T, Westerhausen A, Constantinou C, Prockop DJ (1991) Substitutions for glycine α1–637 and glycine α2–694 of type I procollagen in lethal osteogenesis imperfecta. J Biol Chem 226:15608–15613
Wallis GA, Starman BJ, Zinn AB, Byers PH (1990) Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the α1I gene (COL1A1) of type I collagen in a parent. Am J Hum Genet 46:1034–1040
Wijsman EM (1991) Recurrence risk of a new mutation in children of unaffected parents. Am J Hum Genet 48:654–661
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bonaventure, J., Cohen-Solal, L., Lasselin, C. et al. A dominant mutation in the COL1A1 gene that substitutes glycine for valine causes recurrent lethal osteogenesis imperfecta. Hum Genet 89, 640–646 (1992). https://doi.org/10.1007/BF00221955
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00221955