Summary
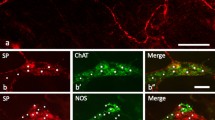
The neuronal subpopulations in the cat stellate, lower lumbar and sacral sympathetic ganglia were studied with regard to the cellular distribution of immunoreactivity to tyrosine hydroxylase (TH), acetylcholinesterase (AChE) and various neuronal peptides. Coexistence of neuropeptide Y (NPY)- and galanin (GAL)-like immunoreactivity (LI) was found in a high proportion of the neuronal cell bodies; these cells also contained immunoreactivity to TH, confirming their presumably noradrenergic nature. Some TH- and GAL-immunoreactive principal ganglion cells lacked NPY-LI. Two populations (scattered and clustered) of vasoactive intestinal polypeptide (VIP)- and peptide histidine isoleucine (PHI)-positive cell bodies were found in the sympathetic ganglia studied. The scattered VIP/PHI neurons also contained AChE-LI, calcitonin gene-related peptide (CGRP)-and, following culture, substance P (SP)-LI. The clustered type only contained AChE-LI. In the submandibular and sphenopalatine ganglia, neurons were AChE- and VIP/ PHI-immunoreactive but lacked CGRP- and SP-LI. Many GAL- and occasional TH-positive neurons were found in these ganglia. In the spinal ganglia, single NPY-immunoreactive sensory neuronal cells were observed, in addition to CGRP- and SP-positive neurons. The present results show that there are at least two populations of sympathetic cholinergic neurons in the cat. Retrograde tracing experiments indicate that the scattered type of cholinergic neurons contains four vasodilator peptides (VIP, PHI, CGRP, SP) and provides an important input to sweat glands, whereas the clustered type (containing VIP and PHI) mainly innervates blood vessels in muscles.
Similar content being viewed by others
References
Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM (1982) Alternative processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 198:240–244
Bentivoglio M, Kuypers HGJM, Catsman-Berrevoets CE, Loewe H, Dann O (1980) Two new fluorescent retrograde neuronal tracers which are transported over long distances. Neurosci Lett 18:25–30
Bohn MC, Kessler JA, Adler JE, Markey K, Goldstein M, Black IB (1984) Simultaneous expression of the SP-peptidergic and noradrenergic phenotypes in rat sympathetic neurons. Brain Res 298:378–381
Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I (1985) Calcitonin gene-related peptide is a potent vasodilator. Nature 313:54–56
Chang MM, Leeman SE, Niall HD (1971) Amino acid sequence of substance P. Nature New Biol 232:86–87
Coons AH (1958) Fluorescent antibody methods. In: Danielli JF (ed) General cytochemical methods. Academic Press, New York, pp 399–422
Cuello AC, Galfre G, Milstein C (1979) Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci USA 76:3532–3536
Darvesh S, Nance DM, Hopkins DA, Armour JA (1987) Distribution of neuropeptide like immunoreactivity in intact and chronically decentralized middle cervical and stellate ganglia of dogs. J Auton Nerv Syst 21:167–180
Ekblad E, Håkanson R, Sundler F, Wahlstedt C (1985) Galaninneuromodulatory and direct contractile effects on smooth muscle preparations. Br J Pharmacol 86:241–246
Fahrenkrug J, Pedersen JH (1984) Development and validation of a specific radioimmunoassay for PHI in plasma. Clin Chim Acta 143:183–192
Fahrenkrug J, Schaffalitzky de Muckadell OB (1977) Radioimmunoassay of vasoactive intestinal polypeptide (VIP) in plasma. J Lab Clin Med 89:1379–1388
Fisher LA, Kikkava DO, Rivier JE, Amara SG, Evans RM, Rosenfeld MG, Vale WW, Brown MR (1983) Stimulation of noradrenergic sympathetic outflow by calcitonin gene-related peptide. Nature 305:534–536
Fisone G, Wu CF, Consolo S, Nordström Ö, Brynne N, Bartfai T, Melander T, Hökfelt T (1987) Galanin inhibits acetylcholine release in the ventral hippocampus of the rat, in vivo and in vitro studies. Proc Natl Acad Sci USA 84:7339–7343
Fredricsson B, Sjöqvist F (1962) A cytomorphological study of cholinesterase in sympathetic ganglia of the cat. Acta Morphol Neerl Scand 47:284–296
Hamberger B, Norberg K-A, Sjöqvist F (1965) Correlated studies on monoamines and acetylcholinesterase in sympathetic ganglia, illustrating the distribution of adrenergic and cholinergic neurons. In: Koelle GB, Douglas WW, Carlsson A (eds) Pharmacology of cholinergic and adrenergic transmission. Pergamon Press, Oxford, pp 41–54
Hartman BK, Zide D, Udenfriend S (1972) The use of dopamine β-hydroxylase as a marker for the noradrenergic pathways of the central nervous system in the rat. Proc Natl Acad Sci USA 69:2722–2726
Heym C, Reinecke M, Weihe E, Forssmann WG (1984) Dopamineβ-hiydroxylase-, neurotensin-, substance P-, vasoactive intestinal polypeptide-, and enkephalin-immunohistochemistry of paravertebral and prevertebral ganglia in the cat. Cell Tissue Res 235:411–418
Holets VR, Hökfelt T, Rökaeus Å, Terenius L, Goldstein M (1988) Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience 24:893–906
Holmstedt B, Sjöqvist F (1957) Distribution of acetocholinesterase in various sympathetic ganglia. Acta Physiol Scand 42 [Suppl] 145:72–73
Holmstedt B, Sjöqvist F (1959) Distribution of acetocholinesterase in the ganglion cells of various sympathetic ganglia. Acta Physiol Scand 47:284–296
Hökfelt T, Kellerth J-O, Nilsson G, Pernow B (1975) Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res 100:235–250
Hökfelt T, Elfvin L-G, Schultzberg M, Goldstein M, Nilsson G (1977) On the occurrence of substance P-containing fibers in sympathetic ganglia: immunohistochemical evidence. Brain Res 132:29–41
Itoh N, Obata K, Yanaihara N, Okamoto H (1983) Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature 304:547–549
Järhult J, Hellstrand P, Sundler F (1980) Immunohistochemical localization and vascular effects of vasoactive intestinal polypeptide in skeletal muscle of the cat. Cell Tissue Res 207:55–64
Kessler JA, Adler J, Bohn M, Black IB (1981) Substance P in sympathetic neurons: regulation by impulse activity. Science 214:335–336
Kessler JA, Adler JE, Bell WO, Black IB (1983) Substance P and somatostatin metabolism in sympathetic and special sensory ganglia in vitro. Neuroscience 9:309–318
Koelle GB (1951) The elimination of enzymatic diffusion artifacts in the histochemical localization of cholinesterases and a survey of their cellular distributions. J Pharmacol Exp Ther 103:153–171
Koelle GB (1955) The histochemical identification of acetylcholinesterase in cholinergic, adrenergic and sensory neurons. J Pharmacol Exp Ther 114:167–184
Koelle GB, Friedenwald JS (1949) A histochemical method for localizing cholinesterase activity. Proc Soc Exp Biol Med 70:617–622
Kummer W (1987) Galanin- and neuropeptide Y-like immunoreac-tivities coexist in paravertebral sympathetic neurons of the cat. Neurosci Lett 78:127–131
Kummer W, Heym C (1988) Neuropeptide distribution in the cervico-thoracic paravertebral ganglia of the cat with particular reference to calcitonin gene-related peptide immunoreactivity. Cell Tissue Res 252:463–471
Landis SC, Fredieu JR (1986) Coexistence of calcitonin gene-related peptide and vasoactive intestinal peptide in cholinergic sympathetic innervation of rat sweat glands. Brain Res 377:177–181
Lindh B, Lundberg JM, Hökfelt T, Elfvin L-G, Fahrenkrug J, Fischer J (1987) Coexistence of CGRP- and VIP-like immunoreactivities in a population of neurons in the cat stellate ganglia. Acta Physiol Scand 131:475–476
Lindh B, Haegerstrand A, Lundberg JM, Hökfelt T, Fahrenkrug J, Cuello AC, Grassi J, Massoulié J (1988) Substance P-, VIP-and CGRP-like immunoreactivities coexist in a population of cholinergic postganglionic sympathetic nerves innervating sweat glands in the cat. Acta Physiol Scand 134:569–570
Lundberg JM, Hökfelt T (1986) Multiple co-existence of peptides and classical transmitters in peripheral autonomic and sensory neurones-functional and pharmacological implications. In: Hökfelt T, Fuxe K, Pernow B (eds) Progress in brain research, Vol 68. Elsevier, Amsterdam, pp 241–262
Lundberg JM, Tatemoto K (1982) Pancreatic polypeptide family (APP, BPP, NPY and PYY) in relation to α-adrenoceptor-resistant sympathetic vasoconstriction. Acta Physiol Scand 116:393–402
Lundberg JM, Hökfelt T, Schultzberg M, Uvnäs-Wallensten K, Köhler C, Said SI (1979) Occurrence of vasoactive intestinal polypeptide (VIP)-like immunoreactivity in certain cholinergic neurons of the cat. Evidence from combined immunohistochemistry and acetylcholinesterase staining. Neuroscience 4:1539–1559
Lundberg JM, Änggård A, Fahrenkrug J, Hökfelt T, Mutt V (1980) Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: Functional significance of coexisting transmitters for vasodilation and secretion. Proc Natl Acad Sci USA 77:1651–1655
Lundberg JM, Änggård A, Fahrenkrug J (1981) Complementary roles of vasoactive intestinal peptide (VIP) and acetylcholine for cat submandibular gland blood flow and secretion. II Effects of cholinergic antagonists and VIP antiserum. Acta Physiol Scand 113:329–336
Lundberg JM, Änggård A, Fahrenkrug J (1982a) Complementary role of vasoactive intestinal polypeptide (VIP) and acetylcholine for cat submandibular blood flow and secretion. III Effects of local infusions. Acta Physiol Scand 114:329–338
Lundberg JM, Änggård A, Fahrenkrug J, Lundgren C, Holmstedt B (1982b) Co-release of VIP and acetylcholine in relation to blood flow and salivary secretion in cat submandibular salivary gland. Acta Physiol Scand 115:525–528
Lundberg JM, Hedlund B, Bartfai T (1982c) Vasoactive intestinal polypeptide (VIP) enhances muscarinic ligand binding in cat submandibular salivary gland. Nature 295:147–149
Lundberg JM, Tcrenius L, Hökfelt T, Martling CR, Tatemoto K, Mutt V, Polak J, Bloom SR, Goldstein M (1982d) Neuropcptidc Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand 116:477–480
Lundberg JM, Terenius L, Hökfelt T, Goldstein M (1983) High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett 42:167–172
Lundberg JM, Terenius L, Hökfelt T, Tatemoto K (1984) Comparative immunohistochemical and biochemical analysis of pancreatic polypeptide-like peptides with special reference to presence of neuropeptide Y in central and peripheral neurons. J Neurosci 4:2376–2386
Markey KA, Kondo S, Shenkman L, Goldstein M (1980) Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol 17:79–85
Marsh D, Grassi J, Vigny M, Massoulié J (1984) An immunological study of rat acetylcholinesterase: comparison with acetylcholinesterases from other vertebrates. J Neurochem 43:204–213
Melander T, Hökfelt T, Rökaeus Å (1986) Distribution of galaninlike immunoreactivity in the rat central nervous system. J Comp Neurol 248:475–517
Melander T, Hökfelt T, Rökaeus Å, Cuello AC, Oertel W, Verhofstad A, Goldstein M (1987) Coexistence of galanin-like immu-noreactivity with catecholamines, 5-hydroxytryptamine, GAB and neuropeptides in the rat CNS. J Neurosci 6:3640–3654
Mutt V, Said SI (1974) Structure of the porcine vasoactive intestinal octacosapeptide: The amino-acid sequence. Use of kallikrein in its determination. Eur J Biochem 42:581–589
Nordström Ö, Melander T, Hökfelt T, Bartfai T, Goldstein M (1987) Evidence for an inhibitory effect of the peptide galanin on dopamine release from the rat median eminence. Neurosci Lett 73:21–26
Ohhashi T, Jacobowitz M (1985) Galanin potentiates electrical stimulation and exogenous norepinephrine-induced contractions in the rat vas deferens. Regul Pept 12:163–171
Pernow B(1983) Substance P. Pharmacol Rev 35:85–141
Rökaeus Å (1987) Galanin — a newly isolated biologically active neuropeptide. TINS 10:158–164
Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM (1983) Production of a novel neuropeptide by the calcitonin gene via tissue-specific RNA processing. Nature 304:129–132
Said SI, Mutt V (1970) Polypeptide with broad biological activity. Isolation from small intestine. Science 169:1217–1218
Scheibner T, Read DJC, Sullivan CE (1988) Distribution of substance P-immunoreactive structures in the developing cat carotid body. Brain Res 453:72–78
Schmitt M, Kummer W, Heym C (1988) Calcitonin gene-related peptide (CGRP)-immunoreactive neurons in the human cervico-thoracic paravertebral ganglia. J Chem Neuroanat 1:287–292
Schmued LC, Fallon JH (1986) Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res 377:147–154
Semenenko FM, Cuello AC, Goldstein M, Lee KY, Sidebottom E (1986) A monoclonal antibody against tyrosine hydroxylase: Application in light and electron microscopy. J Histochem Cytochem 34:817–821
Sjöqvist F (1962) Cholinergic sympathetic ganglion cells. M.D. Thesis, Karolinska Institutet, Stockholm
Skofitsch G, Jacobowitz DM (1985) Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides 6:509–546
Strömberg I, Björklund H, Melander T, Rökaeus Å, Hökfelt T, Olson L (1987) Galanin-immunoreactive nerves in the rat iris: alterations induced by denervations. Cell Tissue Res 250:267–275
Tatemoto K (1982) Neuropeptide Y: complete amino-acid sequence of the brain peptide. Proc Natl Acad Sci USA 79:5485–5489
Tatemoto K, Mutt V (1981) Isolation and characterization of the intestinal peptide porcine PHI (PHI-27), a new member of the glucagon-secretin family. Proc Natl Acad Sci USA 78:6603–6607
Tatemoto K, Carlquist M, Mutt V (1982) Neuropeptide Y: a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296:659–660
Tatemoto K, Rökaeus Å, Jörnvall H, McDonald MJ, Mutt V (1983) Galanin — a novel biologically active peptide from porcine intestine. FEBS Lett 164:124–128
Tramu G, Pillez A, Leonardelli J (1978) An efficient method of antibody elution for the successive or simultaneous localization of two antigens by immunocytochemistry. J Histochem Cytochem 26:322–324
Tschopp FA, Tobler FH, Fischer JA (1984) Calcitonin gene-related peptide in the human thyroid, pituitary and brain. Mol Cell Endocrinol 36:53–57
von Euler US, Gaddum JH (1931) An unidentified depressor substance in certain tissue extracts. J Physiol (Lond) 72:74–87
Zamboni L, de Martino C (1967) Buffered picric acid formaldehyde: A new rapid fixative for electron microscopy. J Cell Biol 35:148A
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lindh, B., Lundberg, J.M. & Hökfelt, T. NPY-, galanin-, VIP/PHI-, CGRP- and substance P-immunoreactive neuronal subpopulations in cat autonomic and sensory ganglia and their projections. Cell Tissue Res. 256, 259–273 (1989). https://doi.org/10.1007/BF00218883
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00218883