Abstract
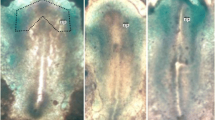
We examined the effects of transforming growth factor-β1 (TGFβ1) and a neutralizing monoclonal antibody on two phases of early chick embryo development: gastrulation and chondrogenesis. We carried out experiments in vivo and in vitro on mesoderm cells from the gastrulating embryo at day 1, and on sclerotome cells from day 3 embryos, having previously shown that this factor is present among these cells at these stages of development. Addition of the antibody to cultures of these cells produced a dose-dependent decrease in cell outgrowth and spreading and concomitantly reduced fibronectin deposition. In vivo studies of the effects of TGFβ1 on mesoderm during gastrulation were carried out by grafting beads carrying this agent into gastrulating embryos. We used beads of ion-exchange resin as well as hydrolysed polyacrylamide, and found that the grafts produced an accumulation of mesoderm cells around the implant and, at later stages, the formation of enlarged somites. There was no effect on embryonic axis formation. Studies of bromodeoxyuridine (BrdU) incorporation indicated that the mesoderm accumulation was due, at least in part, to an increase in cell proliferation. However, examination of the effect of TGFβ1 on BrdU incorporation by mesoderm during gastrulation and sclerotome cells in vitro indicated in inhibition of cell proliferation, an inconsistency explained in terms of the variation between the in vivo and in vitro conditions. We conclude that TGFβ1 is both appropriately located, and is able, to influence cell proliferation among the mesodermal cell populations during early development, and that this effect contributes to the overall control of mesodermal morphogenesis. Chondrogenesis was studied in vitro using micromass cocultures of sclerotome cells with notochordon a permeable substratum. Under these conditions, the addition of TGFβ1 caused an increase in the deposition of Alcian blue-stainable material, indicating a stimulation of chondrogenesis. We suggest that this result, coupled with the previous demonstration that TGFβ1 is present among the sclerotome cells in the embryo at this time, supports the contention that this factor exerts a regulatory effect on sclerotome cell differentiation.
Similar content being viewed by others
References
Barnard JA, Lyons RM, Moses HL (1990) The cell biology of transforming growth factor β. Biochim Biophys Acta 1032:79–87
Bellairs R (1986) The primitive streak. Anat Embryol 174:1–14
Bellairs R, Sanders EJ, Lash JW (1992) Formation and differentiation of early embryonic mesoderm. Plenum Press, New York
Carrington JL, Reddi AH (1990) Temporal changes in the response of chick limb bud mesodermal cells to transforming growth factor β-type 1. Exp Cell Res 186:368–373
Cheney CM, Lash JW (1981) Diversification within embryonic chick somites: differential response to notochord. Dev Biol 81:288–298
Choy M, Armstrong MT, Armstrong PB (1990) Regulation of proliferation of embryonic heart mesenchyme: role of transforming growth factor-β1 and the interstitial matrix. Dev Biol 141:421–425
Cooke J, Wong A (1991) Growth-factor-related proteins that are inducers in early amphibian development may mediate similar steps in amniote (bird) embryogenesis. Development 111:197–212
Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L (1989) Monoclonal antibodies recognizing transforming growth factor-β. J Immunol 142:1536–1541
Eskandarani HA, Ayad SR (1989) Morphological transformation and chemotactic migration of fibroblasts by TGF-β-related 25 Kd polypeptide. Anticancer Res 9:709–714
Godin I, Wylie CC (1991) TGFβ 1 inhibits proliferation and has a chemotropic effect on mouse primordial germ cells in culture. Development 113:1451–1457
Galéra P, Rédini F, Vivien D, Bonaventure J, Penfornis H, Loyau G, Pujol J-P (1992) Effect of transforming growth factor-β1 (TGF-β1) on matrix synthesis by monolayer cultures of rabbit articular chondrocytes during the dedifferentiation process. Exp Cell Res 200:379–392
Hall BK (1977) Chondrogenesis of the somitic mesoderm. Adv Anat 53:1–47
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Hayamizu TF, Sessions SK, Wanek N, Bryant SV (1991) Effects of localized application of transforming growth factor β1 on developing chick limbs. Dev Biol 145:164–173
Jakowlew SB, Dillard PJ, Winokur TS, Flanders KC, Sporn MB, Roberts AB (1991) Expression of transforming growth factor-βs 1–4 in chicken embryo chondrocytes and myocytes. Dev Biol 143:135–148
Joyce ME, Roberts AB, Sporn MB, Bolander ME (1990) Transforming growth factor-β and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol 110:2195–2207
Kulyk WM, Rodgers BJ, Greer K, Kosher RA (1989) Promotion of embryonic chick limb cartilage differentiation by transforming growth factor-β. Dev Biol 135:424–430
Leonard CM, Fuld HM, Frenz DA, Downie SA, Massagué J, Newman SA (1991) Role of transforming growth factor-β in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenous TGF-β and evidence for endogenous TGF-β-like activity. Dev Biol 145:99–109
Lucas PA, Caplan AI (1988) Chemotactic response of embryonic limb bud mesenchymal cells and muscle-derived fibroblasts to transforming growth factor-β. Connect Tissue Res 18:1–7
Massagué J (1990) The transforming growth factor-β family. Ann Rev Cell Biol 6:597–641
Migdalska A, Molineux G, Demuynck H, Evansa GS, Ruscetti F, Dexter TM (1991) Growth inhibitory effects of transforming growth factor-β1 in vivo. Growth Factors 4:239–245
Mitrani E, Shimoni Y (1990) Induction by soluble factors of organized axial structures in chick epiblasts. Science 247:1092–1094
Mitrani E, Ziv T, Thomsen G, Shimoni Y, Melton DA, Bril A (1990) Activin can induce the formation of axial structures and is expressed in the hypoblast of the chick. Cell 63:495–501
Moses HL, Coffey RJ, Leof EB, Lyons RM, Keski-Oja J (1987) Transforming growth factor β regulation of cell proliferation. J Cell Physiol [Suppl 5]: 1–7
Moses HL, Yang EY, Pietenpol JA (1990) TGF-β stimulation and inhibition of cell proliferation: new mechanistic insights. Cell 63:245–247
New DAT (1955) A new technique for the cultivation of the chick embryo in vitro. J Embryol Exp Morphol 3:320–331
Nilsen-Hamilton M (1990) Transforming growth factor-β and its actions on cellular growth and differentiation. Curr Top Dev Biol 24:95–136
Osmond MK, Butler AJ, Voon FCT, Bellairs R (1991) The effects of retinoic acid on heart formation in the early chick embryo. Development 113:1405–1417
Paulsen DF, Solursh M (1988) Microtiter micromass cultures of limb-bud mesenchymal cells. In Vitro Cell Dev Biol 24:138–147
Rizzino A (1988) Transforming growth factor-β: multiple effects on cell differentiation and extracellular matrices. Dev Biol 130:411–422
Roberts AB, Flanders KC, Heine UI, Jakowlew S, Kondaiah P, Kim S-J, Sporn MB (1990) Transforming growth factor-β: multifunctional regulator of differentiation and development. Philos Trans R Soc Lond [Biol] 327:145–154
Rogers SL, Gegick PJ, Alexander SM, McGuire PG (1992) Transforming growth factor-β alters differentiation in cultures of avian neural crest-derived cells: effects on cell morphology, proliferation, fibronectin expression, and melanogenesis. Dev Biol 151:192–203
Sanders EJ (1986) Mesoderm migration in the early chick embryo. In: Browder L (ed) Developmental biology. A comprehensive synthesis, vol 2. Plenum Press, New York, pp 449–480
Sanders EJ (1989) The cell surface in embryogenesis and carcinogenesis. Telford Press, Caldwell, New Jersey
Sanders EJ (1992) Roles for TGFβ1 in chick embryo cell transformation. In: Bellairs R, Sanders EJ, Lash JW (eds) Formation and differentiation of early embryonic mesoderm. Plenum Press, New York, pp 251–262
Sanders EJ, Prasad S (1991) Possible roles for TGFβ1 in the gastrulating chick embryo. J Cell Sci 99:617–626
Seyedin SM, Rosen DM, Segarini PR (1988) Modulation of chondroblast phenotype by transforming growth factor-beta. Pathol Immunopathol Res 7:38–42
Silberstein GB, Daniel CW (1987) Reversible inhibition of mammary gland growth by transforming growth factor-β. Science 237:291–293
Stern CD, Ireland GW, Herrick SE, Gherardi E, Gray J, Ferryman M, Stoker M (1990) Epithelial scatter factor and development of the chick embryonic axis. Development 110:1271–1284
Sutton AB, Canfield AE, Schor SL, Grant ME, Schor AM (1991) The response of endothelial cells to TGFβ-1 is dependent upon cell shape, proliferative state and the nature of the substratum. J Cell Sci 99:777–787
Tang N, Cunningham K, Enger MD (1991) TGFβ elicits opposite responses in clonal subpopulations of NRK-49F cells. Exp Cell Res 196:13–19
Thorp BH, Anderson I, Jakowlew SB (1992) Transforming growth factor-βl, -β2 and-β3 in cartilage and bone cells during endochondral ossification in the chick. Development 114:907–911
Tickle C, Lee J, Eichele G (1985) A quantitative analysis of the effect of all-trans-retinoic acid on the pattern of chick wing development. Dev Biol 109:82–95
Wedden S, Thaller C, Eichele G (1990) Targeted slow-release of retinoids into chick embryos. Methods Enzymol 190:201–210
Yang EY, Moses HL (1990) Transforming growth factor β1-induced changes in cell migration, proliferation, and angiogenesis in the chicken chorioallantoic membrane. J Cell Biol 111:731–741
Zackson SL, Steinberg MS (1989) Axolotl pronephric duct cell migration is sensitive to phosphatidylinositol-specific phospholipase C. Development 105:1–7
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sanders, E.J., Prasad, S. & Hu, N. The involvement of TGFβ1 in early avian development: gastrulation and chondrogenesis. Anat Embryol 187, 573–581 (1993). https://doi.org/10.1007/BF00214436
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00214436