Summary
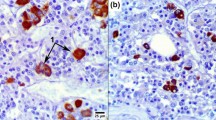
We have demonstrated an extensive reorganization of organ elles in mammotrophs immediately following administration of ergocristine (a dopamine agonist) to estradiol-primed male rats. Our ultrastructural findings are consistent with our previous results that ergocristine can block prolactin release without any noticeable latent period. Following three-week priming of male rats with estradiol implants, ergocristine was administered by a bolus injection through an indwelling cannula. Within two min of its administration, ergocristine induced dramatic changes in the ultrastructure of mammotrophs, i.e., (1) increased numbers of secretory granules, (2) peripheral relocation of rough endoplasmic reticulum which tends to sequester secretory granules, (3) change in location of nucleus and (4) increased numbers of intracellular bodies associated with secretory granules. We suggest that the extensive ultrastructural changes that occurred in such a short period following ergocristine administration may be indications of specific factors associated with blockage of hormone release.
Similar content being viewed by others
References
Antakly T, Pelletier G, Zeytinoglu F, Labrie F (1980) Changes of cell morphology and prolactin secretion induced by 2-Br-α-ergocryptine, estradiol, and thyrotropin-releasing hormone in rat anterior pituitary cells in culture. J Cell Biol 86:377–387
Chen CL, Meites J (1970) Effects of estrogen and progesterone on serum and pituitary levels in ovariectomized rats. Endocrinology 86:503–505
Clemente F, Virgilis G (1967) Ultrastructure de l'adénohypophyse après ovariectomie et traitement par les oestrogènes et la progestérone. Pathol Biol 15:119–131
Duve C de (1963) The lysosome concept. In: AVS de Reuck, MP Cameron (eds) Ciba foundation symposium on lysosomes. Little Brown and Co., Boston, pp 1–35
Duve C de (1964) From cytases to lysosomes. Fed Proc 23:1045–1052
Ectors FA, Danguy A, Pasteels JL (1972) Ultrastructure of organ culture of rat hypophyses exposed to ergocornine. J Endocrinol 52:211–212
Everett JW (1954) Luteotropic function of autografts of the rat hypophysis. Endocrinology 54:685–690
Häusler A, Hodel C (1975) Morphological observations on pituitary mammotrophés of lactating rats treated with 2-bromo-α-ergocryptine (CB-154). Acta Endocrinol suppl 193:66
Hymer WC, McShan WH, Christiansen RG (1961) Electron microscopic studies of anterior pituitary gland from lactating and estrogen-treated rats. Endocrinology 69:81–90
Kalra PS, Fawcett CP, Krulich L, McCann SM (1973) The effects of gonadal steroids on plasma gonadotropins and prolactin in the rat. Endocrinology 92:1256–1268
Krulich L, Hefco EP, Illner P, Read CB (1974) The effects of acute stress on the secretion of LH, FSH, and prolactin and GH in the normal male rat, with comments on their statistical evaluation. Neuroendocrinology 16:293–311
Labrie F, Beaulieu M, Caron MG, Raymond L (1978) The adenohypophyseal dopamine receptor: specificity and modulation of its activity by estradiol. In: C Robyn, M Harter (eds) Progress in prolactin physiology and pathology. Elsevier North Holland Inc, New York, pp 121–136
Lewis PR, Knight DP (1977) Metal precipitation methods for hydrolytic enzymes. In: AM Glauert (ed) Staining methods for sectioned material. North-Holland Press, New York, pp 159–162
MacLeod RM (1976) Regulation of prolactin secretion. In: L Martini, WF Ganong (eds) Frontiers in neuroendocrinology. Vol. IV, Raven Press, New York, pp 169–194
MacLeod RM, Lehmeyer JE (1974) Studies on the mechanism of the dopamine-mediated inhibition of prolactin secretion. Endocrinology 94:1077–1085
McComb DJ, Ryan N, Horváth E, Kovács K, Domolcas I, Laszlo FA (1979) An immunohistochemical and ultrastructural comparison of the effects of 2-bromo-α-ergocryptine on intrasellar and transplanted rat pituitaries. Experientia 35:1409–1410
Müller EE, Panerai AE, Cocchi D, Mantegazzu P (1977) Endocrine profile of ergot alkaloids. Life Sci 21:1545
Novikoff AB (1973) Lysosomes: a personal account. In: HG Hers, F Van Hoof (eds) Lysosomes and storage diseases. Academic Press, New York, pp 1–41
Pantić V, Genbačev O (1969) Ultrastructure of pituitary lactotrophic cells of oestrogen treated male rats. Z Zellforsch 95:280–289
Pantić V, Genbačev O (1972) Pituitaries of rats treated with oestrogen. I. Luteotrophic and somatotrophic cells and hormones content. Z Zellforsch 126:41–52
Piercy M, Shin SH (1980) Comparative studies of prolactin secretion in estradiol-primed and normal male rats induced by ether stress, pimozide and TCH. Neuroendocrinology 31:270–275
Pino RM, Pino LC, Bankston PW (1981) The relationship between the Golgi apparatus GERL, and lysosomes of fetal rat liver Kupffer cells examined by ultrastructural phosphatase cytochemistry. J Histochem Cytochem 29:1061–1070
Reynolds EJ (1963) The use of lead citrate at high pH as an electron-opaque stain for electron microscopy. J Cell Biol 17:208–213
Rossi GL (1978) Effects of 2-bromo-α-ergocryptine on the ultrastructure of anterior pituitaries in different experimental conditions. Pharmacology 16, Suppl 1:193–203
Shin SH (1979a) Effect of ergocristine on prolactin secretion in the male rat with pituitaries grafted beneath the kidney capsule. Can J Physiol Pharmacol 57:1313–1316
Shin SH (1979b) Estradiol generates pulses of prolactin secretion in castrated male rats. Neuroendocrinology 29:270–275
Shin SH, Aiken RB, Roberts R, Howitt C (1974) Effect of testosterone on serum prolactin in the castrated rat. J Endocrinol 63:257–258
Shin SH, Reifel CW (1981) Adenohypophysis has an inherent property for pulsatile prolactin secretion. Neuroendocrinology 32:139–144
Smith RE, Farquhar MG (1966) Lysosome function in the regulation of the secretory process in cells of the anterior pituitary gland. J Cell Biol 31:319–347
Steele RGD, Torrie JH (1960) Principles and procedures of statistics. McGraw-Hill, New York, pp 57–59, pp 107–109
Talwalker PK, Ratner A, Meites J (1963) In vitro inhibition of pituitary prolactin synthesis and release by hypothalamic extracts. Am J Physiol 205:213–218
Weiner RI, Ganong WF (1978) Role of brain monoamines and histamine in regulation of anterior pituitary secretion. Physiol Rev 58:905
West B, Dannies PS (1980) Effects of estradiol on prolactin production and dihydroergocryptine-induced inhibition of prolactin in primary cultures of rat pituitary cells. Endocrinology 106:1108–1113
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reifel, C.W., Shin, S.H. & Leather, R.A. Extensive ultrastructural changes in rat mammotrophs following administration of the dopamine agonist ergocristine-reflecting inhibition of prolactin release. Cell Tissue Res. 232, 249–256 (1983). https://doi.org/10.1007/BF00213784
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00213784