Abstract
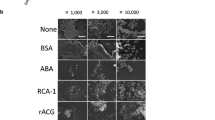
The growth of Streptococcus mutans 6715-13 in a rich medium (Todd Hewitt broth) was drastically reduced by addition of apo-lactoferrin (apo-Lf); this effect was bacteriostatic and reversible by saturation of Lf with iron. The influence of Lf, salivary proteins (SP) and bovine serum albumin (BSA) on the attachment of Streptococcus mutans to hydroxyapatite (HA) was successively investigated. Sorption of Lf, SP, and BSA to HA was dependent on the protein concentration and reached the end-point at about 80 mg of proteins per gram of HA. Similarly, the number of streptococci adsorbed to HA was correlated to the amount of cells available up to at least 107 cells per mg of HA. The adsorption of Lf, SP and BSA on HA reduced the number of attaching S. mutans cells. In particular, SP reduced the adsorption of S. mutans by 30%, whereas pre-coating of HA with apoor iron-saturated Lf resulted in a three orders of magnitude reduction of S. mutans adsorption to HA, as demonstrated by means of different experimental procedures. The powerful adherence-inhibiting effect of apo-Lf together with its noticeable antibacterial activity towards S. mutans points to a biological significance of these phenomena also in vivo.
Similar content being viewed by others
References
Appelbaum B, Golub E, Holt SC, Rosan B (1979) In vitro studies of dental plaque formation; adsorption of oral streptococci to hydroxyapatite. Infect Immun 25:717–728
Arnold RR, Cole NF, McGhee JR (1977) A bactericidal effect for human lactoferrin. Science 197:263–265
Arnold RR, Brewer M, Gauthier JJ (1980) Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect Immun 28:893–898
Arnold RR, Russell JE, Champion WJ, Gauthier JJ (1981) Bactericidal activity of human lactoferrin: influence of physical conditions and metabolic state of the target microorganism. Infect Immun 32:655–660
Arnold RR, Russell JE, Champion WJ, Brewer M, Gauthier JJ (1982) Bactericidal activity of human lactoferrin: differentiation from the stasis of iron deprivation. Infect Immun 35:792–799
Bennick A, Chan G, Goodlin R, Abrams S, Tustian D, Madapallimatan G (1983) The role of human salivary acidic prolin-rich proteins “in vivo” and their fate after adsorption to the human enamel surface. Arch Oral Biol 28:19–27
Bowen WH (1969) The induction of rampant dental caries in monkeys (Macaca irus). Caries Res 3:227–237
Brock JH (1985) The transferrins. In: Harrison P (ed) Metalloproteins, part II. MacMillan, London, pp 183–262
Bullen JJ, Griffiths E (1987) Iron and infection. J Wiley & Sons, Chichester New York Brisbane Toronto Singapore
Ciardi JE, Rolla G, Bowen WH, Reilly JA (1977) Adsorption of Streptococcus mutans lipoteichoic acid to hydroxyapatite. Scand J Dent Res 85:387–391
Clark WB, Gibbons RJ (1977) Influence of salivary components and extracellular polysaccaride synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surface. Infect Immun 18:514–523
Costerton JW, Marrie TJ, Cheng KJ (1985) Phenomena of bacterial adhesion. In: Savage DC, Fletcher M (eds) Bacterial adhesion. Mechanisms and physiological significance. Plenum Press, New York London, pp 3–43
Fine DH, Wilton JMA, Caravana C (1984) “In vitro” sorption of albumin, immunoglobulin G and lysozyme, to enamel and cementum from human teeth. Infect Immun 44:332–338
Fitzgerald RJ, Jordan HV, Stanley HR (1960) Experimental caries and gengival pathologic changes in gnotobiotic rat. J Dent Res 39:923–935
Gibbons RJ (1977) Adherence of bacteria to host tissues. In: Schlessinger D (ed) Microbiology 1977. American Society for Microbiology, Washington, DC, pp 395–406
Gibbons RJ, Houten J van (1973) On the formation of dental plaques. J Periodontol 144:347–360
Gibbons RJ, Houten J van (1975) Bacterial adherence in oral microbial ecology. Microbiol Rev 29:19–44
Hamada S, Torii M (1978) Effect of sucrose in culture media on the location of glucosyltrasferase of Streptococcus mutans and cell adherence to glass surfaces. Infect Immun 20:592–599
Kohler B, Krasse B, Carlen A (1981) Adherence and Streptococcus mutans infection: in vitro study with saliva from noninfected and infected preschool children. Infect Immun 34: 633–636
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193:265–275
Muratsu K, Morioka T (1985) Levels of salivary lysozyme, lactoperoxidase and lactoferrin in diabetic hamsters. Infect Immun 48:389–394
Musaka H, Slade HD (1973) Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell-wall polysaccharide and antigen in plaque formation. Infect Immun 8:555–562
Orsi N, Valenti P, Visca P (1988) Attività antimicrobica delle trasferrine e ruolo di esse nell' immunità naturale. Ig Mod 89:612–626
Pruitt KM (1977) Macromolecular components of oral fluids at tooth surface. Swed Dent J 1:225–240
Pruitt KM, Caldwell RC, Jamieson AD, Taylor RE (1969) The interaction of salivary proteins with tooth surface. J Dent Res 48:818–823
Rudney JD, Smith QU (1985) Relationships between levels of lysozyme, lactoferrin, salivary peroxidase and secretory immunoglobulin A in stimulated parotid saliva. Infect Immun 49:469–475
Staat RH, Langley SD, Doyle RJ (1980) Streptococcus mutans adherence: presumptive evidence for protein-mediated attachment by glucan-dependent cellular accumulation. Infect Immun 27:675–681
Tenovuo J, Lehtonen OJ, Aaltonen AS, Vilja P, Tuohimaa P (1986) Antimicrobial factors in whole saliva of human infants. Infect Immun 51:49–53
Valenti P, Visca P, Antonini G, Orsi N (1985) Interaction between lactoferrin and ovotransferrin and Candida cells. FEMS Microbiol Lett 33:271–275
Zinner DD, Jablon JM, Aran AP, Saslaw MS (1965) Experimental caries induced in animals by streptococci of human origin. Proc Soc Exsp Biol Med 118:766–770
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Visca, P., Berlutti, F., Vittorioso, P. et al. Growth and adsorption of Streptococcus mutans 6715-13 to hydroxyapatite in the presence of lactoferrin. Med Microbiol Immunol 178, 69–79 (1989). https://doi.org/10.1007/BF00203302
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00203302