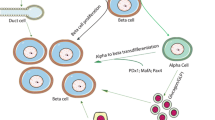
Abstract
The beta cell population of the endocrine pancreas may expand by either of two processes, neogenesis or replication. While replication requires the existence of an already differentiatied beta cell, neogenesis depends on the presence of active stem cells. Since the replicative activity of highly specialized cells such as beta cells seems to be limited, it is interesting to study the potential of the endocrine pancreas for beta cell neogenesis. In this article we review both the current state of knowledge of beta cell neogenesis under natural conditions and in response to stimulation, and the significance of neogenesis for beta cell growth in health and disease.
Similar content being viewed by others
References
Ar'Rajab A, Ahrén B (1993) Long-term diabetogenic effect of streptozotocin in rats. Pancreas 8:50–57
Atkinson MA, Maclaren NK (1994) The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med 331:1428–1436
Beattie GM, Levine F, Mally MI, Otokonski T, O'Brien JS, Salomon DR, Hayek A (1994) Acid β-galactosidase: a developmentally regulated marker of endocrine cell precursors in the human fetal pancreas. J Clin Endocrinol Metab 78:1232–1240
Bertelli E, Regoli M, Bastianini A (1994) Endocrine tissue associated with the pancreatic ductal system: a light and electron microscopic study of the adult rat pancreas with special reference to a new endocrine arrangement. Anat Rec 239:371–378
Bisgaard HC, Thorgeirsson S (1991) Evidence for a common cell of origin for primitive epithelial cells isolated from rat liver and pancreas. J Cell Physiol 147:333–343
Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE (1993) A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes 42:1715–1720
Bouwens L, Wang RN, De Blay E, Pipeleers DG, Klöppel G (1994) Cytokeratins as markers of ductal cell differentiation and islet neogenesis in the neonatal rat pancreas. Diabetes 43:1279–1283
Bouwens L, Braet F, Heimberg H (1995) Identification of rat pancreatic duct cells by their expression of cytokeratins 7, 19 and 20 in vivo and after isolation and culture. J Histochem Cytochem 43:245–253
Campbell IL, Hobbs MV, Dockter J, Oldstone MBA, Allison J (1994) Islet inflammation and hyperplasia induced by the pancreatic islet-specific overexpression of interleukin-6 in transgenic mice. Am J Pathol 145:157–166
Cantenys D, Portha B, Dutrillaux MC, Hollande E, Rozé C, Picon L (1981) Histogenesis of the endocrine pancreas in newborn rats after destruction by streptozotocin. Virchows Arch [B] 35:109–122
De Krijger RR, Aanstoot HJ, Kranenburg G, Reinhard M, Visser WJ, Bruining GJ (1992) The midgestational human fetal pancreas contains cells coexpressing islet hormones. Dev Biol 153:368–375
De Vroede MA, In't Veld PA Pipeleers DG (1990) Deoxyribonucleic acid synthesis in cultured adult rat pancreatic B cells. Endocrinology 127:1510–1516
Dudek RW, Lawrence IE, Hill RS, Johnson RC (1991) Induction of islet cytodifferentiation by fetal mesenchyme in adult pancreatic ductal epithelium. Diabetes 40:1041–1048
Eriksson U, Swenne I (1982) Diabetes in pregnancy: growth of the fetal pancreatic B-cells in the rat. Biol Neonate 42:239–248
Finegood DT, Tobin BW, Lewis JT (1992) Dynamics of glycemic normalization following transplantation of incremental islet masses in streptozotocin-diabetic rats. Transplantation 53:1033–1037
Finegood DT, Scaglia L, Bonner-Weir S (1995) Dynamics of beta-cell mass in the growing rat pancreas, estimation with a simple mathematical model. Diabetes 44:249–256
Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS (1986) The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia 29:267–274
Gepts W, De Mey J (1978) Islet cell survival determined by morphology. An immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes 27 [Suppl 1]:251–261
Gu D, Sarvetnick N (1993) Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development 118:33–46
Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CVE, Teitelman G (1995) Expression of murine STF-1, a putative insulin gene transcription factor, in β-cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development 121:11–18
Hellerström C (1984) The life story of the pancreatic B cell. Diabetologia 26:393–400
Herrera P-L, Huarte J, Sanvito F, Meda P, Orci L, Vassalli J-D (1991) Embryogenesis of the murine endocrine pancreas: early expression of pancreatic polypeptide gene. Development 113:1257–1265
Herrera P-L, Huarte J, Zufferey R, Nichols A, Mermillod B, Philippe J, Muniesa P, Sanvito F, Orci L, Vassalli J-D (1994) Ablation of islet endocrine cells by targeted expression of hormone-promoter-driven toxigenes. Proc Natl Acad Sci USA 91:12999–13003
Higuchi Y, Herrera P, Muniesa P, Huarte J, Belin D, Ohashi P, Aichele P, Orci L, Vassalli JD, Vassalli P (1992) Expression of a tumour necrosis factor α-transgene in murine pancreatic β-cells results in severe and permanent insulitis without evolution towards diabetes. J Exp Med 176:1719–1731
Hultquist GT, Karlsson U, Hallner AC (1979) The regenerative capacity of the pancreas in duct-ligated rats. Exp Pathol 17:44–52
Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, Merlino GT (1990) TGFα-overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell 61:1137–1146
Kanaka-Gantenbein C, Tazi A, Czernichow P, Scharfmann R (1995) In vivo presence of the high affinity nerve growth factor receptor Trk-A in the rat pancreas: differential localization during pancreatic development. Endocrinology 136:761–769
Kanaka-Gantenbein C, Dicou E, Czernichow P, Scharfmann R (1995) Presence of nerve growth factor and its receptors in an in vitro model of islet cell development: implication in normal islet morphogenesis. Endocrinology 136:3154–3162
Kaung HC (1994) Growth dynamics of pancreatic islet cell populations during fetal and neonatal development of the rat. Dev Dyn 200:163–175
Keymeulen B, Teng H, Vetri M, Gorus F, In't Veld PA, Pipeleers DG (1992) Effect of donor islet mass on metabolic normalization in streptozotocin-diabetic rats. Diabetologia 35:719–724
Klöppel G, Bommer G, Commandeur G, Heitz PU (1978) The endocrine pancreas in chronic pancreatitis. Immunocytochemical and ultrastructural studies. Virchows Arch [A] 377:157–174
Klöppel G, In't Veld PA, Stamm B, Heitz PU (1991) The endocrine pancreas. In: Kovacs K, Asa SL (eds) Functional endocrine pathology. Blackwell, Boston, pp 396–457
Larsson LI, Rehfeld JF, Sundler F, Hakanson R (1976) Pancreatic gastrin in foetal and neonatal rats. Nature 262:609–610
Lernmark Å, Klöppel G, Stenger D, Vathanaprida C, Fält K, Landin-Olsson M, Baskin DG, Palmer JP, Gown AM, Petersen JS, Li L, Edenvall H, Mauseth RS (1995) Heterogeneity of islet pathology in two infants with recent onset diabetes mellitus. Virchows Arch 425:631–640
Löhr M, Klöppel G (1987) Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy, Diabetologia 30:757–762
Lomsky R, Langr F, Vortel V (1969) Immunohistochemical demonstration of gastrin in mammalian islets of Langerhans. Nature 223:618–619
Lukinius A, Ericsson JLE, Grimelius L, Korsgren O (1992) Ultrastructural studies of the ontogeny of fetal human and porcine endocrine pancreas, with special reference to colocalization of the four major islet hormones. Dev Biol 153:376–385
Makino T, Usuda N, Rao S, Reddy JK, Scarpelli DG (1990) Transdifferentiation of ductular cells into hepatocytes in regenerating hamster pancreas. Lab Invest 62:552–561
McEvoy RC (1981) Changes in the volumes of the A-, B-, and D-cell populations in the pancreatic islets during the postnatal development of the rat. Diabetes 30:813–817
McEvoy RC, Madson KL (1980) Pancreatic insulin-, glucagon-, and somatostatin-positive islet cell populations during the perinatal development of the rat. I. Morphometric quantitation. Biol Neonate 38:248–254
Müller R, Laucke R, Trimper B, Cossel L (1990) Pancreatic cell proliferation in normal rats studied by in vivo autoradiography with 3H-thymidine. Virchows Arch [B] 59:133–136
Oberg C, Waltenberger J, Claesson-Welsh L, Welsh M (1994) Expression of protein tyrosine kinases in islet cells: possible role of the Flk-1 receptor for β-cell maturation from duct cells. Growth Factors 10:115–126
Otokonski T, Beattie GM, Rubin JS, Lopez AD, Baird A, Hayek A (1994) Hepatocyte growth factor/scatter factor has insulinotropic activity in human fetal pancreatic cells. Diabetes 43:947–953
Pang K, Mukonoweshuro C, Wong GC (1994) Beta cells arise from glucose transporter type 2 (Glut2)-expressing epithelial cells of the developing rat pancreas. Proc Natl Acad Sci USA 91:9559–9563
Pictet R, Rutter WJ (1972) Development of the embryonic endocrine pancreas. In: Geiger SR (ed) Handbook of physiology, section 7: Endocrinology, vol 1. Waverley Press, Baltimore, pp 25–66
Pipeleers D, Keymeulen B, Korbutt G (1994) Islet transplantation. In: Marshall SM, Home PD (eds) The diabetes annual/8. Elsevier, Amsterdam, pp 299–330
Pipeleers-Marichal MA, Pipeleers DG, Cutler J, Lacy PE, Kipnis DM (1976) Metabolic and morphologic studies in intraportal-islet-transplanted rats. Diabetes 25:1041–1051
Rao S, Dwivedi RS, Yeldandi AV, Subbarao V, Tan X, Usman MI, Thangada S, Nemali MR, Kumar S, Scarpelli DG, Reddy JK (1989) Role of periductal and ductular epithelial cells of the adult rat pancreas in pancreatic hepatocyte lineage. A change in the differentiation commitment. Am J Pathol 134:1069–1086
Rosenberg L, Vinik AI (1992) Trophic stimulation of the ductular-islet cell axis: a new approach to the treatment of diabetes. In: Vinik AI (ed) Pancreatic islet cell regeneration and growth. Plenum Press, New York, pp 95–104
Rosenberg L, Clas D, Duguid W (1990) Trophic stimulation of the ductal/islet cell axis: a new approach to the treatment of diabetes. Surgery 108:191–197
Rutter WJ, Wessels NK, Grobstein C (1964) Control of specific synthesis in the developing pancreas. Natl Cancer Inst Monogr 13:51–65
Sacchi TB, Bani D, Biliotti G (1985) Nesidioblastosis and islet changes related to endogenous hypergastrinemia. Virchows Arch [B] 48:261–276
Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC (1990) Overexpression of TGF-α in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell 61:1121–1135
Sanvito F, Herrera PL, Huarte J, Nichols A, Montesano R, Orci L, Vassalli JD (1994) TGF-β-1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development 120:3451–3462
Sarraf C, Lalani EN, Golding M, Anilkumar TV, Poulsom R, Alison M (1994) Cell behavior in the acetylaminofluorene-treated regenerating rat liver. Light and electron microscopic observations. Am J Pathol 145:1114–1126
Swenne I (1983) Effects of aging on the regenerative capacity of the pancreatic B-cell of the rat. Diabetes 32:14–19
Swenne I, Eriksson U (1982) Diabetes in pregnancy: islet cell proliferation in the fetal rat pancreas. Diabetologia 23:525–528
Teitelman G, Lee JK (1987) Cell lineage analysis of pancreatic islet cell development: glucagon and insulin cells arise from catecholaminergic precursors present in the pancreatic duct. Dev Biol 121:454–466
Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D (1993) Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development 118:1031–1039
Upchurch BH, Aponte GW, Leiter AB (1994) Expression of peptide YY in all four islet cell types in the developing mouse pancreas suggests a common peptide YY-producing progenitor. Development 120:245–252
Wang RN, Bouwens L, Klöppel G (1994) Beta-cell proliferation in normal and streptozotocin-treated newborn rats: site, dynamics and capacity. Diabetologia 37:1088–1096
Wang RN, Klöppel G, Bouwens L (1995) Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 38:1405–1411
Wang RN, Bouwens L, Klöppel G (1996) Beta-cell growth in adolescent and adult rats treated with streptozotocin during the neonatal period. Diabetologia (in press)
Wang TC, Bonner-Weir S, Oates PS, Chulak MB, Simon B, Merlino GT, Schmidt EV, Brand SJ (1993) Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J Clin Invest 92:1349–1356
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bouwens, L., Klöppel, G. Islet cell neogenesis in the pancreas. Vichows Archiv A Pathol Anat 427, 553–560 (1996). https://doi.org/10.1007/BF00202885
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00202885