Abstract
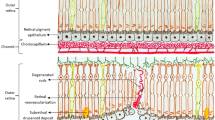
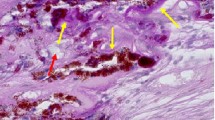
• Background: The morphological features of angiogenesis in early choroidal neovascularization secondary to age-related macular degeneration are yet to be fully described. • Methods: Six eyes from five patients which on clinical and histological examination showed advanced age-related macular degeneration and early choroidal neovascularization have been studied by transmission electron microscopy. • Results: Pre-existing choroidal capillaries and venules showed changes which included endothelial cell budding, pericyte enlargement, endothelial cell sprout formation and the development of intrachoroidal new vessels. In one case, an endothelial cell sprout continuous with an intrachoroidal vessel penetrated Bruch's membrane. Examination of early subretinal pigment epithelial new vessels showed them to spread between the inner layers of Bruch's membrane within the space usually occupied by the basal linear deposit and drusen. New vessel formation took place in blind pouches at the margins of new vessel networks, either in the absence of pericytes or in the presence of mainly myofibroblast-like pericytes. • Conclusion: This ultrastructural study describes two phases of new vessel growth associated with the onset of choroidal neovascularization secondary to age-related macular degeneration. The initial intrachoroidal phase appears to be a “low-turnover” form of neovascularization which may lead to new vessels penetrating Bruch's membrane. Extensive subretinal pigment epithelial neovascularization, on the other hand, results from a “high-turnover” phase of neovascularization characterized by extensive endothelial cell proliferation and migration. Pericyte phenotypic changes associated with these different phases of neovascularization appear to relate to the dynamics of angiogenesis taking place in each process.
Similar content being viewed by others
References
Albrecht-Buehler G (1976) Filipodia of spreading 3T3 cells. Do they have a substrate-exploring function? J Cell Biol 169:275–286
Archer DB, Gardiner TA (1981) Electron microscopic features of experimental choroidal neovascularization. Am J Ophthalmol 91:433–457
Ausprunk DH, Folkman J (1977) Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumour angiogenesis. Microvasc Res 14:53–65
Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR (1994) Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina 14:130–142
Cliff WJ (1963) Observations on healing tissue: a combined light and electron microscopic investigation. Philos Trans R Soc Lond Ser B (Biol Sci) 246:305–325
Crocker DJ, Murad TM, Geer JC (1970) Role of the pericyte in wound healing. An ultrastructural study. Exp Mol Pathol 13:51–65
Dastgheib K, Green WR (1994) Granulomatous reaction to Bruch's membrane in age-related macular degeneration. Arch Ophthalmol 112:813–818
de Juan E, Humayun MS, Hatchell DL, Wilson D (1989) Histopathology of experimental preretinal neovascularization. Invest Ophthalmol Vis Sci 30:1495–1503
Duvall J, Tso MOM (1985) Cellular mechanisms of resolution of drusen after laser coagulation. An experimental study. Arch Ophthalmol 103:694–703
Ferris FL (1983) Senile macular degeneration: a review of epidemiologic features. Am J Epidemiol 118:132–151
Green WR, Enger C (1993) Age-related macular degeneration histopathologic studies. The 1992 Lorenz E Zim merman Lecture. Ophthalmology 100:1519–1535
Green WR, Key SN (1977) Senile macular degeneration: a histopathological study. Trans Am Ophthalmol Soc LXXV:180–254
Hyman LG, Lilienfeld AM, Ferris FL, Fine SL (1983) Senile macular degeneration: a case-control study. Am J Epidemiol 118:213–227
Ingber DE, Folkman J (1989) Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol 109:317–330
Ishibashi T, Miller H, Orr G, Sorgente N, Ryan SJ (1987) Morphologic observations on experimental subretinal neovascularization in the monkey. Invest Ophthalmol Vis Sci 28:1116–1130
Killingsworth MC, Sarks JP, Sarks SH (1990) Macrophages related to Bruch's membrane in age-related macular degeneration. Eye 4:613–621
Korte GE (1989) Choriocapillaris regeneration in the rabbit. Ultrastructure of new endothelial tube formation. Invest Ophthalmol Vis Sci 30:1938–1950
Nagy JA, Brown LF, Senger DR, Lanir N, Van De Water L, Dvorak AM, Dvorak HF (1988) Pathogenesis of tumour stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim Biophys Acta 948:305–326
Orlidge A, D'Amore PA (1987) Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol 105:1455–1462
Penfold PL, Killingsworth MC, Sarks SH (1985) Senile macular degeneration: the involment of immunocompetent cells. Graefe's Arch Clin Exp Ophthalmol 223:69–76
Ryan SJ (1982) Subretinal neovascularization. Natural history of an experimental model. Arch Ophthalmol 100:1804–1809
Ryan SJ (1989) Subretinal neovascularization. In: Ryan SJ, Schachat AP, Murphy RB, Patz A (eds) Retina. Mosby, St. Louis, pp 107–125
Sarks JP, Sarks SH, Killingsworth MC (1994) Evolution of soft drusen in age-related macular degeneration. Eye 8:269–283
Sarks SH (1980) Council Lecture: Drusen and their relationship to senile macular degeneration. Aust J Ophthalmol 8:117–130
Sarks SH, van Driel D, Maxwell L, Killingsworth MC (1980) Softening of drusen and subretinal neovascularization. Trans Ophthalmol Soc UK 100:414–422
Sarks SH, Killingsworth MC, Penfold PL, van Driel D (1985) Patterns in macular degeneration. In: Ryan SJ, Dawson AK, Little HL (eds) Retinal diseases. Grune and Stratton, New York, pp 87–93
Sato N, Sawasaki Y, Senoo A, Fuse Y, Hirano Y, Goto T (1987) Development of capillary networks from rat microvascular fragments in vitro: the role of myofibroblastic cells. Microvasc Res 33:194–210
Schlingemann RO, Rietveld FJR, de Waal RMW, Ferrone S, Ruiter DJ (1990) Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumours and in healing wounds. Am J Pathol 136:1393–1405
Schlingemann RO, Rietveld FJR, Kwaspen F, van de Kerkhof PCM, de Waal RMW, Ruiter DJ (1991) Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol 136:1335–1347
Sholley MM, Gimbrone MA, Cotran RS (1977) Cellular migration and replication in endothelial regeneration. Lab Invest 36:18–25
Sims DE (1986) The pericyte — a review. Tissue Cell 18:153–174
Small L, Green WR, Alpar JJ, Dewry RE Jr (1976) Senile macular degeneration. Clinicopathologic correlation of two cases with neovascularization beneath the retinal pigment epithelium. Arch Ophthalmol 94:601–607
Yamagami I (1970) Electron microscopic study on the cornea. 1. The mechanism of experimental new vessel formation. Jpn J Ophthalmol 14:41–58
Yamamoto T, Yamashita H (1990) Pseudopodia of capillary endothelium in ocular tissues. Jpn J Ophthalmol 34:181–187
Young RW (1987) Pathophysiology of age-related macular degeneration. Surv Ophthalmol 31:291–306
Zhu Z, Goodnight R, Sorgente N, Ogden TE, Ryan SJ (1989) Morphological observations of retinal pigment epithelial proliferation and neovascularization in the rabbit. Retina 9:319–327
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Killingsworth, M.C. Angiogenesis in early choroidal neovascularization secondary to age-related macular degeneration. Graefe's Arch Clin Exp Ophthalmol 233, 313–323 (1995). https://doi.org/10.1007/BF00200479
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00200479