Summary
Acoustic communication in Gryllus firmus is temperature-coupled: temperature induces parallel changes in male calling song temporal pattern, and in female preference for song. Temperature effects on song production and recognition networks were localized by selectively warming head or thorax or both head and thorax of intact crickets, then eliciting aggression song production (males) or phonotaxis to synthetic calling song (females). Because male song is produced by a thoracic central pattern generator (CPG), and because head ganglia are necessary for female song recognition, measurements of female phonotaxis under such conditions may be used to test the following competing hypotheses about organization of the song recognition network: 1. A set of neurons homologous to the male song CPG exist in the female, and are used as a template that determines preferred values of song temporal parameters for song pattern recognition (the common neural elements hypothesis), and 2. temporal pattern preference is determined entirely within the head ganglia.
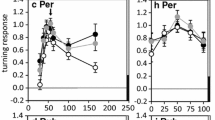
Neither selective warming of the head nor of the thorax was effective in changing female song preference, but simultaneous warming of head and thorax shifted preference toward a faster song in most preparations, as did warming the whole animal by raising ambient temperature. These results suggest that phonotactic preference for song temporal pattern is plurisegmentally determined in field crickets.
Selective wanning experiments during aggression song production in males revealed that syllable period is influenced but not completely determined by thoracic temperature; head temperature is irrelevant. The song CPG appears to receive some rate-setting information from outside the thoracic central nervous system.
Similar content being viewed by others
Abbreviations
- AS :
-
aggression song
- CP :
-
chirp period
- CPG :
-
central pattern generator
- CS :
-
calling song
- SP :
-
syllable period
References
Alexander RD (1962) Evolutionary change in cricket acoustical communication. Evolution 16:443–467
Altner H, Loftus R (1985) Ultrastructure and function of insect thermo- and hygro-receptors. Annu Rev Entomol 30:273–295
Atkins S, Atkins G, Rhodes M, Stout JF (1989) Influence of syllable period on song encoding properties of an ascending auditory interneuron in the cricket Acheta domestica. J Comp Physiol A 165:825–834
Bauer M, Heiversen O von (1987) Separate localization of sound recognizing and sound producing neural mechanisms in a grasshopper. J Comp Physiol A 161:95–101
Bentley DR (1969a) Intracellular activity in cricket neurons during generation of song patterns. Z Vergl Physiol 62:267–283
Bentley DR (1969b) Intracellular activity in cricket neurons during the generation of behavior patterns. J Insect Physiol 15:677–699
Bentley DR (1977) Control of cricket song patterns by descending interneurons. J Comp Physiol 116:19–38
Bentley DR, Hoy RR (1970) Postembryonic development of adult motor patterns in crickets: a neural analysis. Science 170:1409–1411
Bentley DR, Kutsch W (1966) The neuromuscular mechanism of stridulation in crickets (Orthoptera: Gryllidae). J Exp Biol 45:151–164
Boake CRB (1991) Coevolution of senders and receivers of sexual signals: genetic coupling and genetic correlations. Trends Ecol Evol 6:225–227
Butlin RK, Ritchie MG (1989) Genetic coupling in mate recognition systems: what is the evidence? Biol J Linn Soc 37:237–246
Doherty JA (1985a) Temperature coupling and “trade-off” phenomena in the acoustic communication system of the cricket, Gryllus bimaculatus De Geer (Gryllidae). J Exp Biol 114:17–35
Doherty JA (1985b) Trade-off phenomena in calling song recognition and phonotaxis in the cricket, Gryllus bimaculatus (Orthoptera, Gryllidae). J Comp Physiol A 156:787–801
Doherty JA (1991) Song recognition and localization in the phonotaxis behavior of the field cricket Gryllus bimaculatus (Orthoptera: Gryllidae). J Comp Physiol A 168:213–222
Doherty JA, Gerhardt HC (1984) Acoustic communication in hybrid treefrogs: sound production by males and selective phonotaxis by females. J Comp Physiol A 154:319–330
Doherty JA, Pires A (1987) A new microcomputer-based method for measuring walking phonotaxis in field crickets (Gryllidae). J Exp Biol 130:425–432
Elliot CJH (1983) Wing hair plates in crickets: physiological characteristics and connections with stridulatory motor neurones. J Exp Biol 107:21–47
Elsner N (1973) The central nervous control of courtship behaviour in the grasshopper Gomphocerippus rufus L. In: Neurobiology of invertebrates (symposium). Hung Acad Sciences, Tihany, Hungary, pp 261–287
Elsner N (1974a) Neuroethology of sound production in gomphocerine grasshoppers. I. Song patterns and stridulatory movements. J Comp Physiol 88:67–102
Elsner N (1974b) Neural economy: bifunctional muscles and common central pattern elements in leg and wing stridulation of the grasshopper Stenobothrus rubicundus Germ. (Orthoptera: Acrididae). J Comp Physiol 89:227–236
Elsner N (1983) A neuroethological approach to the phylogeny of leg stridulation in gomphocerine grasshoppers. In: Huber F, Markl H (eds) Neuroethology and behavioral physiology. Springer, Berlin Heidelberg New York, pp 54–68
Esch H, Huber F, Wohlers DW (1980) Primary auditory neurons in crickets: physiology and central projections. J Comp Physiol 137:27–38
Gerhardt HC, Doherty JA (1988) Acoustic communication in the gray treefrog Hyla versicolor: evolutionary and neurobiological implications. J Comp Physiol A 162:261–278
Hedwig B (1986) On the role in stridulation of plurisegmental interneurons of the acridid grasshopper Omocestus viridulus L. I. Anatomy and physiology of descending cephalothoracic interneurons. J Comp Physiol A 158:413–427
Hennig RM (1989) Neuromuscular activity during stridulation in the cricket Teleogryllus commodus. J Comp Physiol A 165:837–846
Hoy RR (1974) Genetic control of acoustic behavior in crickets. Am Zool 14:1067–1080
Hoy RR (1978) Acoustic communication in crickets: a model system for the study of feature detection. Fed Proc 37:2316–2323
Hoy RR, Paul RC (1973) Genetic control of song specificity in crickets. Science 180:82–83
Hoy RR, Hahn J, Paul RC (1977) Hybrid cricket auditory behavior: Evidence for genetic coupling in animal communication. Science 195:82–84
Huber F (1963) The role of the central nervous system in Orthoptera during the coordination and control of stridulation. In: Busnel RG (ed) Acoustic behavior of animals. Elsevier, Amsterdam London New York, pp 440–488
Janiszewski J, Otto D (1988) Modulation of activity of identified suboesophageal neurons in the cricket Gryllus bimaculatus by local changes in body temperature. J Comp Physiol A 162:739–746
Janiszewski J, Otto D (1989) Responses and song pattern copying of omega-type I-neurons in the cricket, Gryllus bimaculatus, at different prothoracic temperatures. J Comp Physiol A 164:443–450
Kalmring K (1975) The afferent auditory pathway in the ventral cord of Locusta migratoria (Acrididae). I. Synaptic connectivity and information processing among the auditory neurons of the ventral cord. J Comp Physiol 104:103–141
Kutsch W (1969) Neuromuskulare Aktivitat bei verschiedenen Verhaltensweisen von drei Grillenarten. Z Vergl Physiol 63:335–378
Kutsch W, Huber F (1970) Zentrale versus periphere Kontrolle des Gesanges von Grillen (Gryllus campestris). Z Vergl Physiol 67:140–159
Kutsch W, Otto D (1972) Evidence for spontaneous song production independent of head ganglia in Gryllus campestris L. J Comp Physiol 81: 115–119
Michelsen A (1974) Hearing in invertebrates. In: Autrum H (ed) Handbook of sensory physiology V/I. Springer, Berlin Heidelberg New York, pp 389–422
Miles CI (1985) The effects of behaviourally relevant temperatures on mechanosensory neurones of the grasshopper, Schistocerca americana. J Exp Biol 116:121–139
Moiseff A, Pollack GS, Hoy RR (1978) Steering responses of flying crickets to sound and ultrasound: Mate attraction and predator avoidance. Proc Natl Acad Sci USA 75:4052–4056
Möss D (1968) Proprioceptoren im Thorax und Abdomen von Grillen (Orthoptera, Gryllidae). Zool Anz Suppl 31:123–136
Möss D (1971) Sinnesorgane im Bereich des Flügels der Feldgrille (Gryllus campestris L.) und ihre Bedeutung fur die Kontrolle der Singbewegung und die Einstellung der Flügellage. Z Vergl Physiol 73:53–83
Otto D, Weber T (1982) Interneurons descending from the cricket cephalic ganglia that discharge in the pattern of two motor rhythms. J Comp Physiol 148: 209–219
Otto D, Weber T (1985) Plurisegmental neurons of the cricket Gryllus campestris L. that discharge in the rhythm of its own song. J Insect Physiol 31:537–548
Pearce RA, Friesen WO (1985) Intersegmental coordination of the leech swimming rhythm. I. Roles of cycle period gradient and coupling strength. J Neurophysiol 54:1444–1459
Pires A, Hoy RR (1992) Temperature coupling in cricket acoustic communication: I. Field and laboratory studies of temperature effects on calling song production and recognition in Gryllus firmus. J Comp Physiol A 171:69–78
Pfau HK, Koch UT, Möhl B (1989) Temperature dependence and response characteristics of the isolated wing hinge stretch receptor in the locust. J Comp Physiol A 165:247–252
Pollack GS, Hoy RR (1979) Temporal pattern as a cue for speciesspecific calling song recognition in crickets. Science 204:429–432
Prestwich KN, Walker TJ (1981) Energetics of singing in crickets: effect of temperature in three trilling species (Orthoptera: Gryllidae). J Comp Physiol B 143:199–212
Robertson RM, Pearson KG, Reichert H (1982) Flight interneurons in the locust and the origin of insect wings. Science 217:177–179
Ronacher B (1989) Stridulation of acridid grasshoppers after hemisection of thoracic ganglia: evidence for hemiganglionic oscillators. J Comp Physiol A 164:723–736
Ronacher B, Heiversen D von, Heiversen O von (1986) Routes and stations in the processing of auditory directional information in the CNS of a grasshopper, as revealed by surgical experiments. J Comp Physiol A 158:363–374
Ronacher B, Stumpner A (1988) Filtering of behaviourally-relevant temporal parameters of a grasshopper's song by an auditory interneuron. J Comp Physiol A 163:517–523
Rose GJ, Brenowitz EA, Capranica RR (1985) Species specificity and temperature dependency of temporal processing by the auditory midbrain of two species of treefrogs. J Comp Physiol A 157:763–769
Schaffner K-H, Koch UT (1987) Effects of wing campaniform sensilla lesions on stridulation in crickets. J Exp Biol 129: 25–40
Schildberger K (1984) Temporal selectivity of identified auditory neurons in the cricket brain. J Comp Physiol A 155:171–185
Schildberger K, Hörner M (1988) The function of auditory neurons in cricket phonotaxis. I. Influence of hyperpolarization of identified neurons on sound localization. J Comp Physiol A 163:621–631
Skovmand O, Pedersen SB (1983) Song recognition and song pattern in a shorthorned grasshopper. J Comp Physiol 153:393–401
Sperry RW (1950) Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 43:482–489
Stout JF, McGhee RW (1988) Attractiveness of the male Acheta domesticus calling song to females. II. The relative importance of syllable period, intensity, and chirp rate. J Comp Physiol A 164:277–287
Stumpner A, Ronacher B, Heiversen O von (1991) Auditory interneurones in the metathoracic ganglion of the grasshopper Chorthippus biguttulus. II. Processing of temporal patterns of the song of the male. J Exp Biol 158:411–430
Von Heiversen D (1972) Gesang des Männchens und Lautschema des Weibchens bei der Feldheuschrecke Chorthippus biguttulus (Orthoptera, Acrididae). J Comp Physiol 81:381–422
Von Heiversen D, Heiversen O von (1975a) Verhaltensgenetische Untersuchungen am akustischen Kommunikationssystem der Feldheuschrecken (Orthoptera, Acrididae). I. Der Gesang von Artbastarden zwischen Chorthippus biguttulus und Ch. mollis. J Comp Physiol 104:273–299
Von Heiversen D, Heiversen O von (1975b) Verhaltensgenetische Untersuchungen am akustischen Kommunikationssystem der Feldheuschrecken (Orthoptera, Acrididae). II. Das Lautschema von Artbastarden zwischen Chorthippus biguttulus und Ch. mollis. J Comp Physiol 104:301–323
Von Heiversen O (1979) Angeborenes Erkennen akustischer Schlüsselreize. Verh Dtsch Zool Ges 72:42–59
Von Heiversen D, Heiversen O von (1981) Korrespondenz zwischen Gesang und auslösendem Schema bei Feldheuschrecken. Nova Acta Leopold NF 54 Nr. 245:449–462
Walker TJ (1957) Specificity in the response of female tree crickets (Orthoptera, Gryllidae, Oecanthinae) to calling songs of the males. Ann Entomol Soc Am 50:626–636
Weber T, Thorson J (1988) Auditory behavior of the cricket. IV. Interaction of direction of tracking with perceived temporal pattern in split-song paradigms. J Comp Physiol A 163:13–22
Wiese K, Eilts K (1985) Evidence for matched frequency dependence of bilateral inhibition in the auditory pathway of Gryllus bimaculatus. Zool Jb Physiol 89:181–201
Wohlers DW, Huber F (1982) Processing of sound signals by six types of neurons in the prothoracic ganglion of the cricket, Gryllus campestris L. J Comp Physiol 146:161–173
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pires, A., Hoy, R.R. Temperature coupling in cricket acoustic communication. J Comp Physiol A 171, 79–92 (1992). https://doi.org/10.1007/BF00195963
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00195963