Summary
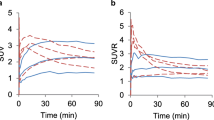
Iron and transferrin are required for DNA synthesis and cell division. Cellular iron uptake is mediated by transferrin receptors. In order to investigate whether iron uptake in brain tumors is associated with their histological grade, we studied 24 patients (5 astrocytoma, 11 glioblastoma, 8 meningioma) using positron emission tomography and 12 Fe-citrate. Tracer uptake from blood into brain and tumor tissue was assessed 1. using multiple time graphical analysis yielding a measure for unidirectional net tracer uptake (Ki and 2.) testing a one- and two-tissue kinetic compartment model, where K1 denotes tracer uptake from blood into tissue, k2 efflux from tissue into plasma, and 0 specific tracer binding. In the plasma, 52Fe was bound to a 80 kD protein (transferrin). Ki (in units of 10−5/min) was higher in glioblastomas (Ki mean ± SD 13.6 ± 6.1) compared with astrocytomas (4.8 ± 3.5, Mann Whitney p = 0.015) and contralateral brain (2.2 ± 0.9, Mann Whitney p = 0.009). Highest values were found in meningiomas (no blood-brain barrier (BBB); Ki 33.4 ± 16.5, Mann Whitney p = 0.008 compared with glioblastomas). Among the compartment models, fitting with Kl1 and regional plasma volume explained the data best (one-tissue model), data fits were not significantly improved by addition of a k2 or k3 parameter. K1 and Ki values were significantly correlated (Spearman Rank, p = 0.0006). We conclude that 52Fe accumulation in tumors is governed by tracer uptake at the BBB, and does not reflect number of transferrin receptors at the level of tumor cells.
Similar content being viewed by others
References
Laskey J, Webb I, Schulman HM, Ponak P: Evidence that transferrin supports cell proliferation by supplying ion for DNA synthesis. Exp Cell Res 176:87–95,1988
Huebers HA, Finch CA: The physiology of transferrin and transferrin receptors. Physiol Rev 67:520–581,1987
Galbraith RM, Galbraith GM: Expression of transferrin receptors on mitogen-stimulated human peripheral blood lymphocytes: Relation to cellular activation and related metabolic events. Immunol 44:703–710,1981
Trowbridge IS, Omary MB: Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci USA 78:3039–3043,1981
Raivich G, Graeber MB, Gehrmann J, Kreutzberg GW: Transferrin receptor expression and iron uptake in the injured and regenerating rat sciatic nerve. Eur J Neurosci 3: 919–927,1991
Trowbridge IS: Transferrin receptor as a potential therapeutic target. In: Waldmann H (ed) Monoclonal antibody therapy. Prog Allergy (Vol. 45). Karger, Basel, 1988, pp 121–146
Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY: Transferrin receptor on endothelium of brain capillaries. Nature 312:162–163,1984
Recht L, Torres CO, Smith TW, Taso V, Griffin TW: Transferrin receptor in normal and neoplastic brain tissue: implications for brain-tumor immunotherapy. J Neurosurg 72: 941–945,1990
Prior R, Reifenberger G, Wechsler W: Transferrin receptor expression in tumours of the human nervous system: relation to tumor type, grading and tumor growth fraction. Virch Arch A Pathol Anat 416:491–496, 1990
Otsuki H, Brunetti A, Owens ES, Finn RD, Blasberg RG: Comparison of iron-59, indium-111, and gallium-69 transferrin as a macromolecular tracer of vascular permeability and the transferrin receptor. J Nucl Med 30:1676–1685,1989
Aisen P: The transferrins. In: Jacobs A, Worwood M (eds) Iron in biochemistry and medicine. Academic Press, London, 1980, pp 87–129
Smith-Jones P, Schwarzbach R, Weinreich R: The production of 52Fe by means of a medium energy proton accelerator. Radiochim Acta 50:33–39,1990
Reist HW, Vacher J: Sensitivity evaluation of positron emission tomographs. Med Progr Technol 17:153–157,1991
Patlak CS, Blasberg RG, Fenstermacher JD: Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metabol 3:1–7, 1983
Patlak CS, Blasberg RG: Graphical evaluation of blood-tobrain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metabol 5: 584–590, 1985
Ralston ML, Jennrich RI: Dud, a derivative-free algorithm for nonlinear least squares. Technometr 20:7–14,1978
Hawkins RA, Phelps ME, Huang SC: Effects of temporal sampling, glucose metabolic rates, and disruptions of the blood-brain barrier on the FDG model with and without a vascular compartment: Studies in human brain tumors with PET. J Cereb Blood Flow Metabol 6:170–183,1986
Leenders KL, Antonini A, Schwarzbach R, Smith-Jones P, Pellikka R, Günther I, Psylla M, Reist H: Blood-to-brain iron transport in man using [52Fe]-citrate and positron emission tomography (PET). In: Uemura K (ed) Quantification of brain function. Tracer kinetics and image analysis in brain PET. Elsevier, Amsterdam, 1993, pp 145–150
Leenders KL, Antonini A, Schwarzbach R, Smith-Jones P, Reist H, Ben-Sacher D, Youdim M, Henn V: Blood to brain iron uptake in one monkey using (52Fe)-citrate and positron emission tomography (PET). J Neur Transm 43:123–132, 1994
Hill J: The distribution of iron in the brain. In: Youdin MBH (ed) Brain iron: Neurochemical and behavioural aspects. Topics in Neurochemistry and Neuropharmacology (Vol 2). Taylor and Francis, London, New York, 1988, pp 1–24
Long DM: Vascular ultrastructure and human meningiomas and schwannomas. J Neurosurg 38:409–419,1973
Nakagawa H, Groothuis DR, Owens ES, Fenstermacher JD, Patlak CS, Blasberg RG: Dexamethasone effects on [125I] albumin distribution in experimental RG-2 gliomas and adjacent brain. J Cereb Blood Flow Metabol 7:687–701, 1987
Johnson V, Wrobel C, Wilson D, Zovickian J, Greenfield L, Oldfield EH, Youle R: Improved tumor-specific immunotoxins in the treatment of CNS and leptomeningeal neoplasia. J Neurosurg 70:240–248,1989
Hall WA, Godal A, Juell S, Fodstad O: In vitro efficacy of transferrin-toxin conjugates against glioblastoma multiforme. J Neurosurg 76: 838–844, 1992
Martell LA, Agrawal A, Ross DA, Muraszko KM: Efficacy of transferrin receptor-targeted immunotoxins in brain tumor cell lines and pediatric brain tumors. Cane Res 53: 1348–1353, 1993
Olsnes S, Sandvik K, Peterson O, van Deurs B: Immunotoxins — entry into cells and mechanisms of action. Immunol. Today 10:291–295,1989
Laske DW, Ilercil O, Akbasak A, Youle RJ, Oldfield EH: Efficacy of direct intratumoral therapy with targeted protein toxins for solid human gliomas in nude mice. J Neurosurg 80:520–526,1994
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roelckel, U., Leenders, K.L., von Ammon, K. et al. Brain tumor iron uptake measured with positron emission tomography and 52Fe-citrate. J Neuro-Oncol 29, 157–165 (1996). https://doi.org/10.1007/BF00182139
Issue Date:
DOI: https://doi.org/10.1007/BF00182139