Summary
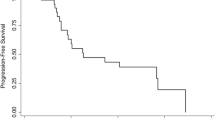
We evaluated the role of gallium nitrate infusion in the treatment of metastatic breast cancer. Gallium nitrate was administered at 300 mg/m2/day for 7 days every 3 weeks by continuous infusion concomitantly with oral calcium supplement of 500 mg twice daily and oral hydration. Fifteen patients with refractory metastatic breast cancer received such treatment for a total of 30 courses. Median age was 51, and median performance status (Zubrod scale) was 1. These patients had minimal prior chemotherapy (median 1 regimen). All patients were evaluable for toxicity and 14 for response. Nine patients had one to two metastatic sites, five patients had three to four sites. No major objective response was seen, but one patient had a minor response (10 weeks), and another showed no change in disease (16 weeks). Diverse low-grade toxicities were observed, including nausea and vomiting in 11 patients, anorexia in 11, diarrhea in eight, stomatitis in five, dysgeusia in six, musculoskeletal pain in five, skin rash in seven, partially reversible tinnitis and/or mild hearing loss in four and sensory neuropathy in two. A consistent drop in hemoglobin (median of 3.2 g/dL per patient) necessitated blood transfusion in seven patients. There was no granulocytopenia or thrombocytopenia; however, significant lymphopenia was noted. Reversible, moderate nephrotoxicity occurred in two patients. The hypocalcemic effect was consistent, with a median drop in serum calcium of 1.25 mg/dL per course. There was no hepatic toxicity. While no single toxicity was severe, overall toxicity adversely influenced treatment tolerance. Gallium nitrate by continuous infusion, as given in this study, has no activity in metastatic breast cancer.
Similar content being viewed by others
References
Hart MM, Adamson RH: Antitumor activity and toxicity of salts of inorganic group IIIa metals: aluminium, gallium, indium, and thallium. Proc Natl Acad Sci USA 68:1623–1626, 1971
Samson MK, Fraile RJ, Baker LH, O'Bryan R: Phase I–II clinical trial of gallium nitrate (NSC-15200). Cancer Clin Trials 3:131–136, 1980
Bedikian AY, Valdivieso M, Bodey GP, Burgess MA, Benjamin RS, Hall S, Freireich EJ: Phase I clinical studies with gallium nitrate. Cancer Treat Rep 62:1449–1453, 1978
Krakoff IH, Newman RA, Goldberg RS: Clinical toxicologic and pharmacologic studies of gallium nitrate. Cancer 44:1722–1727, 1979
Warrell Jr RP, Coonley CJ, Straus DK, Young CW: Treatment of patients with advanced malignant lymphoma using gallium nitrate administered as a seven-day continuous infusion. Cancer 51:1982–1987, 1983
Weick JK, Stephens RL, Baker LH, Jones SE: Gallium nitrate in malignant lymphoma: a Southwest Oncology Group study. Cancer Treat Rep 67:823–825, 1983
Crawford ED, Saiers JH, Baker LH: Treatment of metastatic bladder cancer with gallium nitrate. 13th International Congress of Chemotherapy 240:12.1.7/A4, 84, 1983
Warrell Jr RP, Isaacs M, Coonley CJ, Alcock NW, Bockman RS: Metabolic effects of gallium nitrate administered by prolonged infusion. Cancer Treat Rep 69:653–655, 1985
Warrell Jr RP, Bockman RS, Coonley CJ, Isaacs M, Staszewski D: Gallium nitrate inhibits calcium resorption form bone and is effective treatment for cancer-related hypercalcemia. J Clin Invest 73:1487–1490, 1984
Fabian CJ, Baker LH, Vaughn CB, Hynes HE: Phase II evaluation of gallium nitrate in breast cancer: a Southwest Oncology Group study. Cancer Treat Rep 66:1591, 1982
Warrell Jr RP, Alcock NW, Bockman RS: Gallium nitrate inhibits accelerated bone turnover in patients with bone metastases. J Clin Oncol 5:292–298, 1987
Schwartz S, Yogoda A: Phase I–II trials of gallium nitrate of advanced hypernephroma. Anticancer Res 4:317–318, 1984
Casper ES, Stanton GF, Sordillo PP, Parente R, Michaelson RA, Vinceguerra V: Phase II trial of gallium nitrate in patients with advanced malignant melanoma. Cancer Treat Rep 69:1019–1020, 1985
Vugrin D, Einhorn LH, Birch R: Phase II trial of gallium nitrate in patients with metastatic renal carcinoma. (Abstr) Proc Am Assoc Cancer Res 28:203, 1987
Scher HI, Curley T, Geller N, Dershaw D, Chan E, Nisselbaum J, Alcock N, Hollandere P, Yagoda A: Gallium nitrate in prostatic cancer: evaluation of antitumor activity and effects on bone turnover. Cancer Treat Rep 71:887–893, 1987
Chitamber C, Zivkovic Z, Abrams R: Effects of gallium on hemoglobin production. (Abstr) Proc Am Soc Clin Oncol 5:44, 1986
Saiki JH, Baker LH, Stephens RL, Fabian CJ, Kraut EH, Fletcher WS: Gallium nitrate in advanced soft tissue and bone sarcomas: a Southwest Oncology Group study. Cancer Treat Rep 66:1673–1674, 1982
Decker DA, Costanzi JJ, McCracken JD, Baker LH: Evaluation of gallium nitrate in metastatic or locally recurrent squamous cell carcinoma of the head and neck: a Southwest Oncology Group study. Cancer Treat Rep 68:1047–1048, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jabboury, K., Frye, D., Holmes, F.A. et al. Phase II evaluation of gallium nitrate by continuous infusion in breast cancer. Invest New Drugs 7, 225–229 (1989). https://doi.org/10.1007/BF00170863
Issue Date:
DOI: https://doi.org/10.1007/BF00170863