Abstract
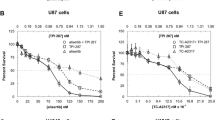
Carboplatin has lower nephro- and neurotoxicities and better penetration into brain tissue than cisplatin. If carboplatin has comparable cytotoxicity against glioma cells, it might have a therapeutic advantage in the treatment of malignant gliomas. Using an assay of colony-forming efficiency, we compared the cytotoxicity of these two drugs in human glioma cell lines SF-126, SF-188, U87-MG, and U251-MG. The experiments were designed so that the product of in vitro drug concentration (C) and time (T) would encompass the same range of values as the C × T of the ultrafilterable platinum plasma fraction as determined by pharmacokinetic studies in man. The in vitro stability of the drugs was evaluated by measuring the cytotoxicity of aged drugs with a microculture tetrazolium assay. Cisplatin and carboplatin were both stable during the 2-h treatment. The cytotoxic activities of these drugs at clinically achievable levels of drug exposure were of the same order of magnitude. These results, in conjunction with the lower nephro- and neurotoxicities of carboplatin, the higher platinum levels in brain tissue after treatment with carboplatin, and the encouraging results of carboplatin in the clinical treatment of brain tumors that have been demonstrated in other studies, suggest that carboplatin might be preferable to cisplatin in the treatment of patients with malignant glioma.
Similar content being viewed by others
References
Shapiro WR, Shapiro JR: Principles of brain tumor chemotherapy. Semin Oncol 13: 56–69, 1986
Walker RW, Allen JC: Cisplatin in the treatment of recurrent childhood primary brain tumors. J Clin Oncol 6: 62–66, 1988
Mahaley MS Jr, Hipp SW, Dropcho EJ, Bertsch L, Cush S, Tirey T, Gillespie GY: Intracarotid cisplatin chemotherapy for recurrent gliomas. J Neurosurg 70: 371–378, 1989
Kornblith PL, Walker M: Chemotherapy for malignant gliomas. J Neurosurg 68: 1–17, 1988
Rosenberg B: Fundamental studies with cisplatin. Cancer 55: 2303–2316, 1985
Vermorken JB, van der Vijgh WJF, Klein I, Gall HE, Pinedo HM: Pharmacokinetics of free platinum species following rapid, 3-hr and 24-hr infusions of cis-diamminedichloroplatinum(II) and its therapeutic implications. Eur J Cancer Clin Oncol 18: 1069–1074, 1982
Litterst CL, LeRoy AF, Guarino AM: Disposition and distribution of platinum following parenteral administration of cis-dichlorodiammineplatinum(II) to animals. Cancer Treat Rep 63: 1485–1492, 1979
DeGregorio M, Wilbur B, King O, Wallenberg J, Prewitt S, Phillips J, Wilbur J: Peak cerebrospinal fluid platinum levels in a patient with ependymoma: evaluation of two different methods of cisplatin administration. Cancer Treat Rep 70: 1437–1438, 1986
Goldie JH, Coldman AJ: Quantitative model for multiple levels of drug resistance in clinical tumors. Cancer Treat Rep 67: 923–931, 1983
Von Hoff DD, Schilsky R, Reichert CM, Reddick RL, Rozencweig M, Young RC, Muggia FM: Toxic effects of cis-dichlorodiammine-platinum(II) in man. Cancer Treat Rep 63: 1527–1531, 1979
Schell MJ, McHaney VA, Green AA, Kun LE, Hayes A, Horowitz M, Meyer WH: Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. J Clin Oncol 7: 754–760, 1989
Fenn LG, Wallace S, Stewart DJ, Chuang VP, Yung WA, Leavens ME, Burgess MA, Savaraj N, Benjamin S, Young SE, Tang RA, Handel S, Mavligit G, Fields WS: Intracarotid infusion of cis-diamminedichloroplatinum in the treatment of recurrent malignant brain tumors. Cancer 54: 794–799, 1984
Olivi A, Duncan KLK, Corden BJ, Lenartz D, Pinn ML, Frye RM, Brem H: Comparison of the CNS toxicity of cisplatin, iproplatin, and carboplatin given by intrathecal administration in a rat model. Proc Am Assoc Can Res 30: 466, 1989 (abstr)
Koeller JM, Trump DL, Tutsch KD, Earhart RH, Davis TE, Tormey DC: Phase I clinical trial and pharmacokinetics of carboplatin (NSC 241240) by single monthly 30-minute infusion. Cancer 57: 222–225, 1986
Muggia FM: Overview of carboplatin: replacing, complementing, and extending the therapeutic horizons of cisplatin. Semin Oncol 16 (Suppl 5): 7–13, 1989
Doz F, Zucker JM, Rosenblum ML: Pharmacokinetics of cisplatin/carboplatin in children. Pediatr Neurosci 14: 164–165, 1988 (abstr)
de Graeff A, Slebos RJC, Rodenhuis S: Resistance to cisplatin and analogues: mechanisms and potential clinical implications. Cancer Chemother Pharmacol 22: 325–332, 1988
Boven E, van der Vijgh WJF, Nauta MM, Schluper MM, Pinedo HM: Comparative activity and distribution of five platinum analogues in nude mice bearing human ovarian carcinoma xenografts. Cancer Res 45: 86–90, 1985
Siddik ZH, Jones M, Boxall FE, Harrap KR: Comparative distribution of carboplatin and cisplatin in mice. Cancer Chemother Pharmacol 21: 19–24, 1988
Alberts DS, Chen HSG, Salmon SE: In vitro drug assay: pharmacological considerations. In: Salmon SE (ed) Cloning of Human Tumor Stem Cells, New York, Alan R Liss, 1980, pp 197–207
Ali-Osman F, Giblin J, Dougherty D, Rosenblum ML: Application of in vivo and in vitro pharmacokinetics for physiologically relevant drug exposure in a human tumor clonogenic cell assay. Cancer Res 47: 3718–3724, 1987
Barker M, Hoshino T, Gurcay O, Wilson CB, Nielsen SL, Downie R, Eliason J: Development of an animal brain tumor model and its response to therapy with 1,3-Bis(2-chloroethyl)-1-nitrosourea. Cancer Res 33: 976–986, 1973
Ponten J, MacIntyre EH: Long-term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand 74: 465–486, 1968
Bigner DD, Bigner SH, Ponten J, Westermark B, Mahaley MS Jr, Ruoslahti E, Herschman H, Eng LF, Wikstrand CJ: Heterogeneity of genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol 40: 201–229, 1981
Rutka JT, Giblin JR, Dougherty DY, Liu HC, McCulloch JR, Bell CW, Stern RS, Wilson CB, Rosenblum ML: Establishment and characterization of five cell lines derived from human malignant gliomas. Acta Neuropathol (Berl) 75: 92–103, 1987
Chen TR: In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res 104: 252–262, 1977
Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB: Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 47: 936–942, 1987
Rosenblum ML, Vasquez DA, Hoshino T, Wilson CB: Development of a clonogenic cell assay for human brain tumors. Cancer 41: 2305–2314, 1978
Berens ME, Giblin JR, Dougherty DV, Hoifodt HK, Tveit K, Rosenblum ML: Comparison of in vitro cloning assays for drug sensitivity testing of human brain tumors. Br J Neurosurg 2: 227–234, 1988
Sasaki Y, Tamura T, Eguchi K, Shinkai T, Fujiwara Y, Fukuda M, Ohe Y, Bungo M, Horichi N, Niimi S, Minato K, Nakagawa K, Saijo N: Pharmacokinetics of (glycolato-0,0′)-diammine platinum(II), a new platinum derivative, in comparison with cisplatin and carboplatin. Cancer Chemother Pharmacol 23: 243–246, 1989
Bajorin DF, Bosl GJ, Alcock NW, Niedzwiecki D, Gallina E, Shurgot B: Pharmacokinetics of cis-diamminedichloroplatinum(II) after administration in hypertonic saline. Cancer Res 46: 5969–5972, 1986
Vermorken JB, van der Vijgh WJF, Klein I, Gall HE, van Groeningen CJ, Hart GAM, Pinedo HM: Pharmacokinetics of free and total platinum species after rapid and prolonged infusions of cisplatin. Clin Pharmacol Ther 39: 136–144, 1986
Reece PA, Stafford I, Abbott RL, Anderson C, Denham J, Freeman S, Morris RG, Gill PG, Olweny CL: Two- versus 24-hour infusion of cisplatin: pharmacokinetic considerations. J Clin Oncol 7: 270–275, 1989
Ozols RF: Optimal dosing with carboplatin. Semin Oncol 16 (Suppl 5):14–18, 1989
Oguri S, Sakakibara T, Mase H, Shimizu T, Ishikawa K, Kimura K, Smyth RD: Clinical pharmacokinetics of carboplatin. J Clin Pharmacol 28: 208–215, 1988
Elferink F, van der Vijgh WJF, Klein I, Vermorken JB, Gall HE, Pinedo HM: Pharmacokinetics of carboplatin after iv administration. Cancer Treat Rep 71: 1231–1237, 1987
Newell DR, Eeles RA, Gumbrell LA, Boxall FE, Horwich A, Calvert AH: Carboplatin and etoposide pharmacokinetics in patients with testicular teratoma. Cancer Chemother Pharmacol 23: 367–372, 1989
Gaver RC, Colombo N, Green MD, George AM, Deeb G, Morris AD, Canetta RM, Speyer JL, Farmen RH, Muggia FM: The disposition of carboplatin in ovarian cancer patients. Cancer Chemother Pharmacol 22: 263–270, 1988
Daley-Yates PT: The metabolites of platinum antitumour drugs and their biological significance. In: McBrien DCH, Slater TF (eds) Biochemical Mechanisms of Platinum Antitumour Drugs, Oxford, IRL Press, pp 121–146, 1986
Knox RJ, Friedlos F, Lydall DA, Roberts JJ: Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diammine-dichloroplatinum(II) and cis-diammine-(1,1-cyclobutane-dicarboxylato) platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res 46: 1972–1979, 1986
Hildebrand-Zanki SU, Kern DH: A rapid bioassay to determine stabilities of anticancer agents under conditions of the clonogenic assay. In Vitro Cell Dev Biol 22: 247–252, 1986
Micetich KC, Barnes D, Erickson LC: A comparative study of the cytotoxicity and DNA-damaging effects of cis-(diammino)(1,1-cyclobutanedicarboxylato)-platinum(II) and cis diamminedichloroplatinum(II) on L1210 cells. Cancer Res 45:4043–4047, 1985
Behrens BC, Hamilton TC, Masuda H, Grotzinger KR, Whang-Peng J, Louie KG, Knutsen T, McKoy WM, Young RC, Ozols RF: Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res 47: 414–418, 1987
Harstrick A, Casper J, Guba R, Wilke H, Poliwoda H, Schmoll HJ: Comparison of the antitumor activity of cisplatin, carboplatin, and iproplatin against established human testicular cancer cell lines in vivo and in vitro. Cancer 63: 1079–1083, 1989
Takahashi H, Sasaki Y, Saijo N, Sakurai M, Nakano H, Nakagawa K, Hoshi A, Jett JR, Hong WS: In vitro colony inhibition of carboplatin against stomach and lung cancer cell lines in comparison with cisplatin. Cancer Chemother Pharmacol 19: 197–200, 1987
Russell J, Adam J, Wheldon TE, Kaye SB: The relative effectiveness of analogues of cisplatin in the experimental chemotherapy of human non-small-cell lung cancer and neuroblastoma grown as multicellular spheroids. Cancer Chemother Pharmacol 23: 111–114, 1989
Fan D, Baker FL, Khokhar AR, Ajani JA, Tomasovic B, Newman RA, Brock WA, Tueni E, Spitzer G: Antitumor activity against human tumor samples of cis-diamminedichloroplatinum(II) and analogues at equivalent in vitro myelotoxic concentrations. Cancer Res 48: 3135–3139,1988
Dodion P, Sanders C, Georges P, Kenis Y: In vitro chemosensitivity of brain tumors to cisplatin and its analogues, iproplatin and carboplatin. Cancer Chemother Pharmacol 22: 80–82, 1988
Nichols CR, Tricot G, Williams SD, van Besien K, Loehrer PJ, Roth BJ, Alkard L, Hoffman R, Goulet R, Wolff SN, Giannone L, Greer J, Einhorn LH, Jansen J: Dose-intensive chemotherapy in refractory germ cell cancer — a phase I/II trial of high dose carboplatin and etoposide with autologous bone marrow transplantation. J Clin Oncol 7: 932–939, 1989
Ozols RF: Cisplatin dose intensity. Semin Oncol 16 (Suppl 6): 22–30, 1989
Allen JC, Walker R, Luks E, Jennings M, Barfoot S, Tan C: Carboplatin and recurrent childhood brain tumors. J Clin Oncol 5: 459–463, 1987
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doz, F., Berens, M.E., Dougherty, D.V. et al. Comparison of the cytotoxic activities of cisplatin and carboplatin against glioma cell lines at pharmacologically relevant drug exposures. J Neuro-Oncol 11, 27–35 (1991). https://doi.org/10.1007/BF00166994
Issue Date:
DOI: https://doi.org/10.1007/BF00166994