Summary
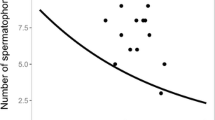
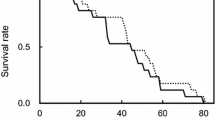
Reproduction for male bushcrickets is energetically expensive. Male Requena verticalis invest 70% of their daily energy reserves in calling to attract a female and providing her with a nutritious spermatophore. Males are thereby likely to be constrained in their mating frequency. I investigated constraints on reproduction imposed by body size and the levels of a protozoan gut parasite when males were fed diets that differed in their nutritional value. Males suffered a cost of reproduction in terms of an increasing interval between matings that was independent of diet and parasitic infection. After three successive matings, males decreased the magnitude of investment in courtship feeding when fed a diet poor in protein. Furthermore, these males suffered a reduction in the number of times they were capable of mating relative to males fed a diet rich in protein. Male size constrained mating frequency on both rich and poor diets; small males were able to mate less frequently than large males. There was an interaction between the effects of diet and parasitic infection on male mating frequency. Heavily infected males mated less frequently than uninfected individuals when fed the poor diet. However, males fed the rich diet were able to overcome the constraints imposed by parasitic infection. Reproductive constraints are discussed in relation to the costs of reproduction and their effects on courtship roles.
Similar content being viewed by others
References
Bailey WJ, Cunningham RJ, Lebel L (1990) Song power, spectral distribution and female phonotaxis in the bushcricket Requena verticalis (Tettigoniidae:Orthoptera): active female choice or passive attraction. Anim Behav 40:33–42
Bailey WJ, Withers PC, Endersby M, Gaull K (in press) The energetic costs of calling in the bushcricket Requena verticalis (Listroscelidinae:Tettigoniidae:Orthoptera). J Exp Biol
Calow P (1979) The cost of reproduction: a physiological approach. Biol Rev 54:23–40
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–222
Fisher RA (1958) The genetical theory of natural selection. Oxford University Press, Oxford
Forbes MRL, Baker RL (1991) Condition and fecundity of the damselfly, Enallagma ebrium (Hagen): the importance of ectoparasites. Oecologia 86:335–341
Gustaffson L, Part T (1990) Acceleration of senescence in the collared flycatcher Ficedula albicollis. Nature 347:279–281
Gustaffson L, Sutherland WJ (1988) The costs of reproduction in the collared flycatcher Ficedula albicollis. Nature 335:813–815
Gwynne DT (1988) Courtship feeding and the fitness of female katydids (Orthoptera:Tettigoniidae). Evolution 42:545–555
Gwynne DT (1990) Testing parental investment and the control of sexual selection in katydids: the operational sex ratio. Am Nat 136:474–484
Gwynne DT, Simmons LW (1990) Experimental reversal of courtship roles in an insect. Nature 346:172–174
Gwynne DT, Bowen BJ, Codd CG (1984) The function of the katydid spermatophore and its role in fecundity and insemination (Orthoptera: Tettigoniidae). Aust J Zool 32:15–22
Halliday TR (1987) Physiological constraints on sexual selection. In: Bradbury JW, Andersson MB (eds) Sexual selection: testing the alternatives. John Wiley & Sons, Chichester, pp 247–264
Hamilton W, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Harry OG (1965) Studies on the early development of the eugregarine Gregarina garnhami. J Protozool 12:296–305
Harry OG (1969) A jar for maintaining parasite free insects and for collecting infected feces. Bull Entomol Res 58:833
MacNally RC, Doolan JM (1982) Comparative reproductive energetics of the sexes in the cicada Cystosoma saundersii. Oikos 39:179–186
Moller AP (1990) Effects of parasitism by a haematophagous mite on reproduction in the barn swallow. Ecology 71:2345–2357
Partridge L, Sibly R (1991) Constraints in the evolution of life histories. Phil Trans R Soc Lond B 332:3–13
Petrie M (1983) Mate choice in role-reversed species. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 167–179
Ryan MJ, Bartholomew GA, Rand AS (1983) Energetics of reproduction in a neotropical frog, Physalaemua pustulosus. Ecology 64:1456–1462
Simmons LW (1988) Male size, mating potential and lifetime reproductive success in the field cricket, Gryllus bimaculatus (de Geer). Anim Behav 36:372–379
Simmons LW, Bailey WJ (1990) Resource influenced sex roles of zaprochiline tettigoniids (Orthoptera:Tettigoniidae). Evolution 44:1853–1868
Simmons LW, Teale RJ, Maier M, Standish RJ, Bailey WJ, Withers PC (1992) Some costs of reproduction for male bushcrickets, Requena verticalis (Orthoptera:Tettigoniidae): allocating resources to mate attraction and nuptial feeding. Behav Ecol Sociobiol 31:57–62
Smyth JD (1976) Introduction to animal parasitology. Hodder and Stoughton, London Stearns SC (1989) Trade-offs in life history evolution. Funct Ecol 3:259–268
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man, 1871–1971. Aldine, Chicago, pp 136–179
Wedell N (1991) Sperm competition selects for nuptial feeding in a bushcricket. Evolution 45:1975–1978
Wedell N (in press) Mating effort or paternal investment: incorporation rate and the cost of male donations in the wartbiter. Behav Ecol Sociobiol
Wedell N, Arak A (1989) The wartbiter spermatophore and its effect on female reproductive output (Orthoptera:Tettigoniidae, Deeticus verrucivorus). Behav Ecol Sociobiol 24:117–125
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am Nat 100:687–690
Zuk M (1987) The effects of gregarine parasites, body size, and time of day on spermatophore production and sexual selection in field crickets. Behav Ecol Sociobiol 21:65–72
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Simmons, L.W. Some constraints on reproduction for male bushcrickets, Requena verticalis (Orthoptera : Tettigoniidae) diet, size and parasite load. Behav Ecol Sociobiol 32, 135–139 (1993). https://doi.org/10.1007/BF00164046
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00164046