Abstract
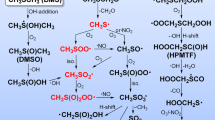
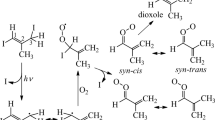
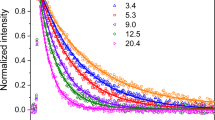
The mechanisms for atmospheric photooxidation of CH3SCH3 and CH3SSCH3 developed in Part I are evaluated by a series of outdoor smog chamber experiments. Measured product yields, including SO2, H2SO4, CH3SO3H and HCHO, are reported. The predictions of the mechanisms developed in Part I are found to be in substantial agreement with the measured concentrations from the smog chamber. By comparison of mechanism predictions and observations, critical uncertainties in the mechanism are identified.
Similar content being viewed by others
References
BallaR. J., NelsonH. H., and McDonaldJ. R., 1986, Kinetics of the reaction of CH3S with NO, NO2 and O2, Chem. Phys. 109, 101–107.
BarnesI., BastianV., BeckerK. H., and NikiH., 1987, FTIR spectroscopic studies of the CH3S+NO2 reaction under atmospheric conditions, Chem. Phys. Lett. 140, 451–457.
BarnesI., BastianV., and BeckerK. H., 1988, Kinetics and mechanisms of the reaction of OH radicals with dimethyl sulfide, Int. J. Chem. Kinet. 20, 415–431.
BensonS. W., 1978, Thermochemistry and kinetics of sulfur-containing molecules and radicals, Chem. Rev. 78, 23–35.
CalvertJ. G. and PittsJ. N.Jr., 1966, Photochemistry, John Wiley, New York.
ChatgilialogluC., GrillerD., and GuerraM., 1987, Experimental and theoretical approaches to the optical absorption spectra of sulfonyl radicals, J. Phys. Chem. 91, 3747–3750.
Daykin, E. P. and Wine, P. H., 1990, A study of the reactions of NO3 radicals with organic sulfides: reactivity trend at 298 K, submitted to Int. J. Chem. Kinet.
DemerjianK. L., SchereK. L., and PetersonJ. T., 1980, Theoretical estimates of actinic (spherically integrated) flux and photolytic rate constants of atmospheric species in the lower troposphere, Adv. Environ. Sci. Technol. 10, 369–459.
DomineF., RavishankaraA. R., and HowardC. J., 1989, Atmospheric reactions of some reduced sulfur radicals, Trans. Am. Geophys. Union 70, 1008.
GrosjeanD. and LewisR., 1982, Atmospheric photooxidation of methyl sulfide, Geophys. Res. Lett. 9, 1203–1206.
GrosjeanD., 1984, Photooxidation of methyl sulfide, ethyl sulfide, and methanethiol, Environ. Sci. Technol. 18, 460–468.
GrosjeanD., 1985, Wall loss of gaseous pollutants in outdoor Teflon chambers, Environ. Sci. Technol. 19, 1059–1065.
HatakeyamaS., OkudaM., and AkimotoH., 1982, Formation of sulfur dioxide and methanesulfonic acid in the photooxidation of dimethyl sulfide in the air, Geophys. Res. Lett. 9, 583–586.
HatakeyamaS. and AkimotoH., 1983, Reactions of OH radicals with methanethiol, dimethyl sulfide, and dimethyl disulfide in air, J. Phys. Chem. 87, 2387–2395.
HatakeyamaS., IzumiK., and AkimotoH., 1985, Yield of SO2 and formation of aerosol in the photo-oxidation of DMS under atmospheric conditions, Atmos. Environ. 19, 135–141.
HynesA. J., WineP. H., and SemmesD. H., 1986, Kinetic and mechanism of OH reactions with organic sulfides, J. Phys. Chem. 90, 4148–4156.
LeoneJ. A., FlaganR. C., GrosjeanD., and SeinfeldJ. H., 1985, An outdoor smog chamber and modeling study of toluene—NO x photooxidation, Int. J. Chem. Kinet. 17, 177–216.
MacLeodH., AschmannS. M., AtkinsonR., TuazonE. C., SweetmanJ. A., WinerA. M., and PittsJ. N.Jr., 1986, Kinetics and mechanisms of the gas phase reactions of the NO3 radical with a series of reduced sulfur compounds, J. Geophys. Res. 91, 5338–5346.
NikiH., MakerP. D., SavageC. M., and BreitenbachL. P., 1983, An FTIR study of the mechanism for the reaction HO+CH3SCH3, Int. J. Chem. Kinet. 15, 647–654.
SternJ. E., FlaganR. C., GrosjeanD., and SeinfeldJ. H., 1987, Aerosol formation and growth in atmospheric aromatic hydrocarbon, Environ. Sci. Technol. 21, 1224–1231.
ToonO. B., KastingJ. F., TurcoR. P., and LiuM. S., 1987, The sulfur cycle in the marine atmosphere, J. Geophys. Res. 92, 943–963.
TyndallG. S. and RavishankaraA. R., 1989, Kinetics and mechanisms of the reactions of CH3S with O2 and NO2 at 298 K, J. Phys. Chem. 93, 2426–2435.
YinF., GrosjeanD., and SeinfeldJ. H., 1990, Mechanism of atmospheric photooxidation of organosulfur compounds, I. Mechanism Development, J. Atmos. Chem. 11, 308–364.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yin, F., Grosjean, D., Flagan, R.C. et al. Photooxidation of dimethyl sulfide and dimethyl disulfide. II: Mechanism evaluation. J Atmos Chem 11, 365–399 (1990). https://doi.org/10.1007/BF00053781
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00053781