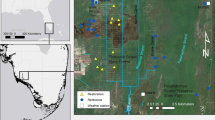
Recent reports have suggested that a global decline in amphibian populations has taken place during the past few decades. Urban development is thought to affect the richness and abundances of species and, therefore, could be an important cause of decline. We estimated the richness and abundances of anurans in wetlands at a residential development and in similar wetlands at a nearby undeveloped park. The residential development originally was pine flatwoods habitat, as is the undeveloped park curiently. We also compared the anuran species' composition of the park in 1992 with the composition in 1974.
Both richness and abundances of anurans in the residential development were different than those in the undeveloped park. Employing the same amount of sampling effort at both sites, we trapped or observed 11 species at the developement and 15 species at the park, and trapped 569 individuals at the development and 1224 individuals at the park. The anuran species richness at the undeveloped park in 1992 was nearly the same as in 1974; a single rare species apparently was not present in 1992. Of the 15 species present in both surveys, 14 showed higher abundances in 1992 than in 1974.
We suggest that the current differences between the residential development and the park have resulted from degradation of both the uplands used by many species during the dry season and the temporary wetlands used by many species for reproduction. Four species especially sensitive to such degradation, Bufo quercicus, Scaphiopus h. holbrookii, Hyla femoralis, and H. gratiosa, were the species missing from the residential development.
Not all species of anurans typical of pine flatwoods appeared to be affected adversely by development. Three species of ranids, Rana utricularia, R. grylio, and R. catesbeiana, were found in higher abundances at the residential development than at the park. These ranid species breed in a wide variety of aquatic systems, including the permanent bodies of water that are now abundant in the development, and probably use the uplands less than other anurans.
If amphibian decline is international in scope, then the decline could be attributable either to global changes caused by humans, or to local, but widespread, environmental degradation, or to a combination of factors. While much recent popular focus has been on potential global causes of decline, we believe that this emphasis may have caused attention to be taken away from local causes that, as our study demonstrated, may be at least as important. We suggest that in many places, local environmental degradation is insidiously chipping away at amphibian diversity, and that more emphasis should be placed on these local causes than is now the case.
Similar content being viewed by others
References
Abrahamson, W.G. and Hartnett, D.C. (1990) Pine flatwoods and dry prairies. In Ecosystems of Florida (R.L. Myers and J.J. Ewel, eds) pp. 103–49. Orlando: University of Central Florida Press.
Andren, C., Henrikson, L., Olsson, M. and Nilson, G. (1988) Effects of pH and aluminium on the embryonic and early larval stages of the Swedish brown frogs Rana arvalis, R. temporaria, and R. dalmantina. Holarct. Ecol. 11, 127–35.
Ashton, R.E. and Ashton, P.S. (1981) Handbook of Reptiles and Amphibians of Florida Part I. Snakes. Miami: Windward.
Ashton, R.E. and Ashton, P.S. (1988) Handbook of Reptiles and Amphibians of Florida Part III. The Amphibians. Miami: Windward.
Auffenberg, W. and Franz, R. (1982) The distribution of the Gopher Tortoise (Gopherus polyphemus). In North American Tortoises: Conservation and Ecology (R.B. Bury, ed.) pp. 95–126. US Dept. Int. Fish Wildl. Serv. Wildlife Research Report 12.
Beebee, T. (1979) Habitat of the British amphibians: 2. Suburban parks and gardens. Biol. Conserv. 15, 157–66.
Beebee, T. (1981) Habitat of the British amphibians: 4. Agricultural lowlands and general discussion of requirements. Biol. Conserv. 21, 127–39.
Behler, J.L. and King, F.W. (1988) The Audubon Society Field Guide to North American Reptiles and Amphibians. New York: Alfred A. Knopf.
Belbin, L. (1992) Comparing two sets of community data: A method for testing reserve adequacy. Aust. J. Ecol. 17, 255–62.
Berven, K.A. and Grudzien, T.A. (1990) Dispersal of the woodfrog (Rana sylvatica): Implications for genetic population structure. Evolution 44, 2047–56.
Blaustein, A.R. (1994a) Chicken Little or Nero's Fiddle? A perspective on declining amphibian populations. Herpetologica 28, 85–97.
Blaustein, A.R. (1994b) Amphibians in a bad light. What is killing the eggs of Oregon's Western Toad? Nat. Hist. 10, 32–9.
Blaustein, A.R. and Wake, D.B. (1990) Declining amphibian populations: A global phenomenon? Trends Ecol. Evol. 5, 203–4.
Blaustein, A.R., Hokit, D.G., O'Hara, R.K. and Holt, R.A. (1994a) Pathogenic fungus contributes to amphibian losses in the Pacific-Northwest. Biol. Conserv. 67, 251–4.
Blaustein, A.R., Wake, D.B. and Sousa, W.P. (1994b) Amphibian declines: judging stability, persistence, and susceptibility of populations to local and global extinction. Conserv. Biol. 8, 60–71.
Boyd, G., and Gardiner, N. (1990) Urban storm water: an overview for municipalities. Public Works 121, 39–42.
Bradford, D.F., Swanson, C. and Gordon, M.S. (1992) Effects of low pH and aluminium on two declining species of amphibians in the Sierra Nevada, California. J. Herpetol. 26, 369–77.
Bureau of Economic Business and Research (1992) Florida Statistical Abstract. Gainesville: University Presses of Florida.
Campbell, H.W. and Christman, S.P. (1982) Field techniques for herpetofaunal community analysis. In Herpetological Communities (N.J. Scott, ed.) pp. 193–200. Washington, D.C.: US Dept Int. Fish Wildlife Service, Wildlife Research Report 13.
Case, T.J. (1991) Invasion resistance, species build-up and community collapse in metapopulation models with interspecific competition. Biol. J. Linn. Soc. 42, 239–66.
Christien, D. and Taylor, D.H. (1978) Population dynamics in breeding aggregations of the American toad, Bufo americanus (Amphibia, Anura, Bufonidae). J. Herpetol. 12, 17–24.
Cochran, P.A. (1988) The herpetofauna of Pheasant Branch and Waunakee Marshes, Dane County, Wisconsin, with a comparison to the intervening agricultural area. Bull. Chic. Herpetol. Soc. 23, 69–71.
Cochran, P.A. (1989) Historical changes in suburban herpetofauna in Du Page County, Illinois. Bull. Chic. Herpetol. Soc. 24, 1–7.
Conant, R. and Collins, J.T. (1991) A Field Guide to Reptiles and Amphibians. Eastern and Central North America. Boston: Houghton Mifflin.
Corn, P.S. (1994) Inventory and monitoring. In Measuring and Monitoring Biological Diversity. Standard Methods for Amphibians (H.R. Heyer, W.R. Heyer, M.A. Donnelli and R.W. McDiarmid, eds) pp. 109–17. Washington: Smithsonian Institution Press.
Corn, P.S. and Bury, R.B. (1989) Logging in western Oregon: Responses of headwater habitats and stream amphibians. For. Ecol. Manage. 29, 39–57.
Cowell, B.C., Burch, D.G., Brown, L.N., McDiarmid, R.W. and Woolfenden, G.E. (1974) Impact assessment of the Lower Hillsborough River Flood Detention Area. Technical Report for the South West Florida Water Management District. Tampa: University of South Florida.
Cox, J. (1992) A cross-tabulation of land cover types by conservation lands in Florida. Fla. Field Nat. 20, 72–5.
Dickman, C.R. (1987) Habitat fragmentation and vertebrate species richness in an urban environment. J. Appl. Ecol. 24, 337–51.
Dodd, C.K. (1990) The status of the red hills salamander Phaeognathus hubrichti, Alabama, USA, 1976–88. Biol. Conserv. 54, 0–19.
Dodd, C.K. (1991) Drift fences associated sampling bias of amphibians at a Florida sandhill temporary pond. J. Herpetol. 25, 296–301.
Dodd, C.K. (1992) Biological diversity of a temporary pond herpetofauna in north Florida sandhills. Biodiv. Conserv. 1, 125–42.
Dodd, C.K. and Charest, B.G. (1988) The herpetofaunal community of ponds in north Florida sandhills: Species composition, temporal use, and management implications. In Management of Amphibians, Reptiles and Small Mammals in North America (R.C. Szaro, K.E. Severson and D.R. Patton, eds) pp. 87–97. F.T. Collins, Colorado US Dept of Agriculture, Forest Service, General Technical Report RM-166.
Duellman, W.E. and Schwartz, A. (1958) Amphibians and reptiles of southern Florida. Bull. Fla. State Mus. Biol. Sci. 3, 181–224.
Duellman, W.E. and Trueb, L. (1986) Biology of Amphibians. New York: MacGraw-Hill.
Dunson, W.A. and Connell, J. (1982) Specific inhibition of hatching in amphibian embryos by low pH. J. Herpetol. 16, 314–16.
Dunson, W.A., Wyman, R.L. and Corbett, E.S. (1992) A symposium on amphibian declines and habitat acidification. J. Herpetol 26, 349–52.
Edenhamn, P., Nilsson, T., Ebenhard, T. and Sjorgren, P. (1992) What is habitat fragmentation for amphibians? Global Ecol. Biogeogr. Lett. 2, 57–9.
Elmberg, J. (1993) Threats to boreal frogs. Ambio 22, 254–5.
Enge, K.M. and Marion, W.R. (1986) Effects of clearcutting and site preparation on herpetofauna on north Florida flatwoods. For. Ecol. Manage. 14, 177–92.
Freda, J. and Dunson, W.A. (1986) Effects of low pH and other chemical variables on the local distribution of amphibians. Copeia 1986, 454–66.
Gilbert, O.L. (1989) The Ecology of Urban Habitats. London: Chapman & Hall.
Gill, D.E. (1978) Effective population size and interdemic migration rates in a metapopulation of the red-spotted newt, Notophthalmus viridescens (Rafinesque). Evolution 32, 839–49.
Hagstrom, T. (1981) Reproductive strategy and success of amphibians in waters acidified by atmospheric pollution. In Proceedings of the European Herpetological Symposium p. 55–7. Burford, England: Cotswold Wild Life Park Ltd.
Hamilton, W.J. (1955) Notes on the ecology of the oak toad in Florida. Herpetologica 11, 205–10.
Hansen, K.L. (1958) Breeding patterns of the eastern spadefoot toad. Herpetologica 14, 57–67.
Hanski, I. (1991) Metapopulation dynamics: brief history and domain. Biol. J. Linn. Soc. 42, 3–16.
Heinen, J.T. (1992) Comparison of the leaf litter herpetofauna in abandoned cacao plantations and primary rain forest in Costa Rica: some implications for faunal restoration. Biotropica 24, 431–9.
Henrikson, L. (1990) Predation on amphibian eggs and tadpoles by common predators in acidified lakes. Holarct. Ecol. 22, 254–5.
Jackson, D.R. and Milstrey, E.G. (1989) The fauna of gopher tortoise burrows. Fla. Game Fresh Water Fish Comm., Nongame Wildl. Prog. Tech. Rep. 5, 86–98.
Jameson, D.L. (1956) Growth, dispersal and survival of the Pacific treefrog. Copeia 1956, 25–9.
Jaskoski, B.J. and Kniders, R.J. (1974) DDT and Methoxychlor in a frog population in Cook County. Transc. st. Ill. Acad. Sci. 67, 341–4.
Jetter, W. and Harris, L.D. (1976) The effect of perturbation on cypress dome animal communities. U. Fla. Cent. Wetl. Annu. Rep. 3, 577–653.
Judd, F.W. (1977) Toxicity of monosodium methaneorsonate herbicide to Couch's spadefoot toad, Scaphiopus couchi. Herpetologica 33, 44–6.
Karns, D.R. (1992) Effects of acidic bog habitats on amphibian reproduction in a northern Minnesota wetland. J. Herpetol. 26, 401–12.
Kautz, R.S. (1993) Trends in Florida wildlife habitat 1936–1987. Fla. Sci. 56, 7–24.
Lannoo, M.J., Lang, K., Waltz, T. and Phillips, G.S. (1994) An altered amphibian assemblage: Dickinson County, Iowa, 70 years after Frank Blanchard's Survey. Am. Midl. Nat. 131, 311–19.
Mann, W., Dorn, P. and Brandl, R. (1991) Local distribution of amphibians: the importance of habitat fragmentation. Global Ecol. Biogeogr. Lett. 1, 36–41.
Martof, B.S. (1953) Territoriality in the green frog Rana clamitans. Ecology 34, 165–74.
McCoy, E.D. (1994) “Amphibian Decline”: a scientific dilemma in more ways than one. Herpetologica 50, 98–103.
Minton, S.A. (1968) The fate of amphibian and reptiles in a suburban area. J. Herpetol. 2, 113–16.
Mohanty-Hejmadi, P. and Dutta, S.K. (1981) Effects of some pesticides on the development of the Indian bull frog Rana tigerina. Environ. Pollut. 24, 145–61.
Moler, P.E. and Franz, R. (1988) Wildlife values of small, isolated wetlands in the southeastern coastal plain. Proc. Southeast Nongame Endang. Wildl. Symp. 3, 234–41.
Mushinsky, H.R. (1985) Fire and the Florida sandhill herpetofaunal community: with special attention to responses of Cnemidophorus sexlineatus. Herpetologica 41, 333–42.
Murphy, C.G., Sherman, P.W. and Emlen, S.T. (1993) Reproductive strategies of the treefrog Hyla gratiosa: Implications for management. Tallahassee. Fla. Game Fresh Wat. Fish Comm.
Myers, R.L. (1990) Scrub and high pine. In Ecosystems of Florida (R.L. Myers and J.J. Ewel) pp. 150–93. Orlando: University of Central Florida Press.
Neill, W.T. (1952) Burrowing habits of Hyla gratiosa. Copeia 1952, 196.
Nichols, J.D. (1992) Capture-recapture methods. BioScience 42, 94–102.
Pechmann, J.H.K. and Wilbur, H.M. (1994) Putting declining amphibian populations in perspective: Natural fluctuations and human impacts. Herpetologica 50, 65–84.
Pechmann, J.H., Scott, D.E., Semlitsch, R.D., Caldwell, J.P., Vitt, L.J. and Gibbons, J.W. (1991) Declining amphibian populations: the problem of separating human impacts from natural fluctuations. Science 253, 892–5.
Peroni, P.A. and Abrahamson, W.G. (1985) Vegetation loss on the southern Lake Wales Ridge, Palmetto 1985, 6–7.
Phillips, K. (1994) Tracking the Vanishing Frogs. An Ecological Mystery. New York: Saint Martin's Press.
Pickett, S.T.A. and White, P.S. (1985) The Ecology of Natural Disturbance and Patch Dynamics. Orlando: Academic Press.
Porter, K.R. and Hakanson, D.E. (1976) Toxicity of mine drainage to embryonic and larval boreal toads (Bufonidae: Bufo boreas). Copeia 1976, 327–31.
Pounds, J.A. and Crump, M.L. (1994) Amphibian declines and climate disturbances: the case of the Golden Toad and the Harlequin Frog. Conserv. Biol. 8, 72–85.
Ranjit Daniels, R.J. (1991) The problems of conserving amphibians in the Western Ghats, India. Curr. Sci. 60, 630–2.
Ritke, M.E., Babb, J.G. and Ritke, M.K. (1991) Breeding-site specificity in the gray treefrog (Hyla chrysoscelis). J. Herpetol. 25, 123–5.
Schroeter, S.C., Dixon, J.D., Kastendiek, J. and Smith, R.O. (1993) Detecting the ecological effects of environmental impacts: a case study of kelp forest invertebrates. Ecol. Appl. 3, 331–50.
Sjorgren, P. (1991) Extinction and isolation gradients in metapopulations: the case of the pool frog (Rana lessonae). Biol. J. Linn. Soc. 42, 135–47.
Solow, A.R. (1993) A simple test for change in community structure. J. Anim. Ecol. 62, 191–3.
Stebbins, R.C. (1951) Amphibians of Western North America. Berkeley: University California Press.
Stewart-Oaten, A., Murdoch, W.W. and Parker, K.E. (1986) Environmental impact assessment: “Pseudoreplication” in time? Ecology 67, 929–40.
Travis, J. (1994) Calibrating our expectations in studying amphibian populations. Herpetologica 50, 104–8.
Vickers, C.R., Harris, L.D. and Swindel, B.F. (1985) Changes in herpetofauna resulting from ditching of cypress ponds in coastal plains flatwoods. For. Ecol. Manage. 11, 17–29.
Whiteley, D. (1977) Amphibian fauna of Sheffield. Sorby Record 15, 36–48.
Wilson, L.D. and Porras, L. (1983) The Ecological Impact of Man on the South Florida Herpetofauna. Univ. Kans. Mus. Nat. Hist. Monogr. No. 9.
Woodward, B.D. (1983) Predator-prey interactions and breeding-pond use of temporary-pond species in a desert anuran community. Ecology 64, 1549–55.
Wright, A.H. and Wright, A.A. (1949) Handbook of Frogs and Toads of the United States and Canada. Ithaca: Coinstock.
Zug, G.R. and Zug, P.B. (1979) The marine toad, Bufo marinus: a natural history resume of native populations. Smithson. Contr. Zool. 284, 1–58.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Delis, P.R., Mushinsky, H.R. & McCoy, E.D. Decline of some west-central Florida anuran populations in response to habitat degradation. Biodivers Conserv 5, 1579–1595 (1996). https://doi.org/10.1007/BF00052117
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00052117