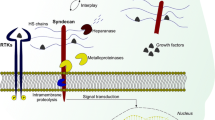
Abstract
There is a growing realization that the whole tumor cell-matrix complex must be investigated in order to fully understand the process of cancer growth and metastasis. Proteoglycans are intrinsic constituents of the cell surface, extracellular matrix, and basement membrane, three logistically and functionally important structures involved in most cellular interactions. Proteoglycans influence the behavior of normal and malignant cells by virtue of their expanded configuration, polyanionic nature and, most of all, by their ability to interact with a variety of cellular products. Consequently, they have been implicated in a number of biological processes including proliferation, recognition, adhesion, and migration. They can serve as links between the extracellular and intracellular environment and thus transduce key biological signals. They can act as receptors for interstitial collagens and other matrix proteins and thus contribute to the organization of pericellular matrix. During neoplastic development there is a profound structural rearrangement of these macromolecules at both the plasma membrane and the pericellular level. Qualitative and quantitative abnormalities in proteoglycan metabolism may contribute to the establishment of some well-known neoplastic properties, including lack of cohesiveness, abnormal assembly of extracellular matrix, abnormal growth, and invasion. The present work will focus on recent advances in our understanding of these complex macromolecules and on some of the alterations associated with the neoplastic phenotype, and will then attempt to elucidate some of the mechanisms regulating these changes.
Similar content being viewed by others
References
Hascall VC, Hascall GK: Proteoglycans. In: Hay ED (ed) Cell Biology of Extracellular Matrix. Plenum Press, New York, 1981, pp 39–63
Heinegård DK, Paulsson M: Structure and metabolism of proteoglycans. In: Piez DA, Reddi AH (eds) Extracellular Biochemistry. Elsevier Publishing Company, New York, 1984, pp 277–328
Iozzo RV: Proteoglycans: Structure, function and role in neoplasia. Lab Invest 53: 373–396, 1985
Iozzo RV: Proteoglycans and the intercellular tumor matrix. In: Seifert G (ed) Current Topics in Pathology. Springer Verlag, Berlin, 1987, pp 207–221
Höök M, Kjellén L, Johansson S, Robinson J: Cell-surface glycosaminoglycans. Ann Rev Biochem 53: 847–869, 1984
Gallagher JT, Lyon M, Steward WP: Structure and function of heparan sulfate proteoglycans. Biochem J 236: 313–325, 1986
Iozzo RV: Cell-surface heparan sulfate proteoglycan and the neoplastic phenotype. J Cell Biochem (in press)
Rapraeger A, Bernfield M: An integral membrane proteoglycan is capable of binding components of the cytoskeleton and the extracellular matrix. In: Hawkes S, Wang J (eds) Extracellular Matrix. Academic Press, New York, 1982, pp 265–269
Rapraeger A, Jalkanen M, Bernfield M: Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol 103: 2683–2696, 1986
Woods A, Höök M, Kjellén L, Smith CG, Rees DA: Relationship of heparan sulfate proteoglycans to the cytoskeleton and extracellular matrix of cultured fibroblasts. J Cell Biol 99: 1743–1753, 1984
Woods A, Couchman JR, Hööks M: Heparan sulfate proteoglycans of rat embryo fibroblasts. A hydrophobic form may link cytoskeleton and matrix components. J Biol Chem 260: 10872–10879, 1985
Carey DJ, Todd MS: Cytoskeleton-associated plasma membrane heparan sulfate in Schwann cells. J Biol Chem 260: 7518–7525, 1986
Carey DJ, Rafferty CM, Schramm MM: Association of heparan sulfate proteoglycan and laminin with the cytoskeleton in rat liver. J Biol Chem 262: 3376–3381, 1987
Fransson L-Å: Self-association of bovine lung heparan sulphates. Identification and characterization of contact zones. Eur J Biochem 120: 251–255, 1981
Fransson L-Å, Havsmark B, Sheehan JK: Self-association of heparan sulfate. Demonstration of binding by affinity chromatography of free chains on heparan sulfate-substituted agarose gels. J Biol Chem 256: 13039–13043, 1981
Fransson L-Å, Carlstedt I, Coster L, Malmstrom A: Proteoheparan sulfate from human skin fibroblasts. Evidence for self-interaction via the heparan sulfate side chains. J Biol Chem 258: 14342–14345, 1983
Stamatoglou SC, Keller JM: Interactions of cellular glycosaminoglycans with plasma fibronectin and collagen. Biochim Biophys Acta 719: 90–97, 1982
Stamatoglou SC, Keller JM: Correlation between cell substrate attachment in vitro and cell surface heparan sulfate affinity for fibronectin and collagen. J Cell Biol 96: 1820–1823, 1983
Koda JE, Bernfield M: Heparan sulfate proteoglycans from mouse mammary epithelial cells. Basal extracellular proteoglycan binds specifically to native Type I collagen fibrils. J Biol Chem 259: 11763–11770, 1984
Koda JE, Rapraeger A, Bernfield M: Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J Biol Chem 260: 8157–8162, 1985
Dietrich CP, Sampaio LO, Toledo OMS, Cassaro CMF: Cell recognition and adhesiveness: a possible biological role for the sulfated mucopolysaccharides. Biochem Biophys Res Commun 75: 329–336, 1977
Chiarugi VP, Vannucchi S, Cella C, Fibbi G, Del Rosso M, Cappelletti R: Intercellular glycosaminoglycans in normal and neoplastic tissues. Cancer Res 38: 4717–4721, 1978
Iozzo RV, Goldes JA, Chen W-J, Wight TN: Glycosaminoglycans of pleural mesothelioma: a possible biochemical variant containing chondroitin sulfate. Cancer 48: 89–97, 1981
Ozzelio L, Lasfargues EY, Murray MR: Grewth-promoting activity of acid mucopolysaccharides on a strain of human mammary carcinoma cells. Cancer Res 20: 600–605, 1960
Takeuchi J: Growth-promoting effect of chondroitin sulfate on solid Ehrlich ascites tumor. Nature (London) 207: 537–538, 1972.
Takeuchi J: Effect of chondroitinase on the growth of solid Ehrlich ascites tumor. Br J Cancer 26: 115–119, 1972
Kraemer PM: Heparan sulfates of cultured cells. I. Membrane-associated and cell sap species in Chinese hamster cells. Biochemistry 10: 1437–1445, 1971
Kraemer PM, Smith DA: High molecular-weight heparan sulfate from the cell surface. Biochem Biophys Res Commun 56: 423–430, 1974
Kraemer PM, Tobey RA: Cell-cycle dependent desquamation of heparan sulfate from the cell surface. J Cell Biol 55: 713–717, 1972.
Chiarugi VP, Vannucchi S: Surface heparan sulphate as a control element in eukariotic cells: a working model. J Theor Biol 61: 459–475, 1976
Culp LA, Rollins BJ, Buniel J, Hitri S: Two functionally distinct pools of glycosaminoglycan in the substrate adhesion sites of murine cells. J Cell Biol 79: 788–801, 1978
Ohnishi T, Ohshima E, Ohtsuka M: Effect of liver cell coat acid mucopolysaccharide on the appearance of density-dependent inhibition in hepatoma cell growth. Exp Cell Res 93: 136–142, 1975
Kawakami H, Terayama H: Liver plasma membranes and proteoglycan prepared therefrom inhibit the growth of hepatoma cells in vitro. Biochim Biophys Acta 646: 161–168, 1981
Castellot JJ, Addonizio ML, Rosenberg R, Karnovsky MJ: Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol 90: 372–379, 1981
Clowes AW, Karnovsky MJ: Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature (London) 265: 625–626, 1977
Castellot JJ, Favreau LV, Karnovsky MJ, Rosenberg RD: Inhibition of vascular smooth muscle cell growth by endothelial cell-derived heparin. Possible role of a platelet endoglycosidase. J Biol Chem 257: 1256–1260, 1982
Mayer K, Chaffee E: Hyaluronic acid in pleural fluid associated with malignant tumor involving pleura and peritoneum. Proc Soc Exp Biol Med 42: 797–800, 1939
Morse BS, Nussbaum M: The detection of hyaluronic acid in the serum and urine of a patient with nephroblastoma. Am J Med 42: 996–1001, 1967
Hopwood JJ, Dorfman A: Glycosaminoglycan synthesis by Wilms' tumor. Pediat Res 12: 52–56, 1978
Powars DR, Allerton SE, Bierle J, Butler BB: Wilms' tumor. Clinical correlation with circulating mucin in three cases. Cancer 29: 1597–1605, 1972
Höpwood JJ, Dorfman A: Glycosaminoglycan synthesis by cultured human skin fibroblasts after transformation with simian virus 40. J Biol Chem 252: 4777–4783, 1977
Angello JC, Danielson KG, Anderson LW, Hosick HL: Glycosaminoglycan synthesis by subpopulations of epithelial cells from a mammary adenocarcinoma. Cancer Res 42: 2207–2210, 1982
Angello JC, Hosick HL, Anderson LW: Glycosaminoglycan synthesis by a cell line (C1-S1) established from a preneoplastic mammary outgrowth. Cancer Res 42: 4975–4978, 1982
Glimelius B, Norling B, Westermark B, Wasteson Å: Composition and distribution of glycosaminoglycans in cultures of human normal and malignant glial cells. Biochem J 172: 443–456, 1978
Pacifici M, Boettiger D, Roby K, Holtzer H: Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell 11: 891–899, 1977
Shanley DJ, Cossu G, Boettiger D, Holtzer H, Pacifici M: Transformation by Rous sarcoma virus induces similar patterns of glycosaminoglycan synthesis in chick embryo skin fibroblasts and vertebral chondroblasts. J Biol Chem 258: 810–816, 1983
Iozze RV: Proteoglycans and neoplastic-mesenchymal cells interactions. Human Path 15: 2–10, 1984
Takeuchi J, Sobue M, Sato E, Shamoto M, Miura K, Nakagaki S: Variation in glycosaminoglycan components of breast tumors. Cancer Res 36: 2133–2139, 1976
Hatae R, Atsuta T, Makita A: Glycosaminoglycans in human lung carcinoma. Gann 68: 59–63, 1977
Kojima J, Nakamura N, Kanatani M, Ohmori K: The glycosaminoglycans in human hepatic cancer. Cancer Res 35: 542–547, 1975
Iozzo RV, Bolender RP, Wight TN: Proteoglycan changes in the intercellular matrix of human colon carcinoma: an integrated biochemical and sterelogic analysis. Lab Invest 47: 124–138, 1982
Iozzo RV, Wight TN: Isolation and characterization of proteoglycans synthesized by human colon and colon carcinoma. J Biol Chem 257: 11135–11144, 1982
De Klerk DP, Lee DV, Human HJ: Glycosaminoglycans of human prostatic cancer. J Urol 131: 1008–1012, 1984
Underhill CB, Keller JM: A transformation-dependent difference in the heparan sulfate associated with the cell surface. Biochem Biophys Res Commun 63: 448–454, 1975
Nakamura N, Hurst RE, West SS: Biochemical composition and heterogeneity of heparan sulfates isolated from AH-130 ascites hepatoma cells and fluid. Biochim Biophys Acta 538: 445–457, 1978
Hurst RE, Parmley RT, Nakamura N, West SS, Denys FR: Heparan sulfate of AH-130 ascites hepatoma cells: a cell-surface glycosaminoglycan not displaced by heparin. J Histochem Cytochem 29: 731–737, 1981
Winterbourne DJ, Mora PT: Cells selected for high tumorigenicity or transformed by simian virus 40 synthesize heparan sulfate with reduced degree of sulfation. J Biol Chem 256: 4310–4320, 1981
Nakamura N, Kojima J: Changes in charge density of heparan sulfate isolated from cancerous human liver tissue. Cancer Res 41: 278–283, 1981
David G, Van Den Berghe H: Transformed mouse mammary epithelial cells synthesize undersulfated basement membrane proteoglycan. J Biol Chem 258: 7338–7344, 1983
Robinson J, Viti M, Höök M: Structure and properties of an undersulfated heparan sulfate proteoglycan synthesized by a rat hepatoma cell line. J Cell Biol 98: 946–953, 1984
Keller KL, Keller JM, Moy JN: Heparan sulfates from Swiss mouse 3T3 and SV3T3 cells: O-sulfate difference. Biochemistry 19: 2529–2536, 1980
Riesenfeld J, Höök M, Lindhal U: Biosynthesis of heparan sulfate: characterization of polysaccharides obtained with intact cells and a cell-free system. J Biol Chem 257: 7050–7055, 1982
Sugahara K, Schwartz NB: Defect in 3′-phosphoadenosine 5′-phosphosulfate formation in brachymorphic mice. Proc Natl Acad Sci USA 76: 6615–6618, 1979
Kajiwara T, Tanzer ML: Undersulfated proteoglycans are induced by the ionopohore monensin: study of possible mechanisms. Arch Biochem Biophys 214: 51–55, 1982
Fransson L-Å, Sjoberg I, Chiarugi VP: Co-polymeric glycosaminoglycans in transformed cells. Transformation-dependent changes in the self-associating properties of cell-surface heparan sulfate. J Biol Chem 256: 13044–13047, 1981
Khoory MS, Nesheim ME, Bowie EJW, Mann KG: Circulating heparan sulfate proteoglycan anticoagulant from a patient with a plasma cell disorder. J Clin Invest 65: 666–674, 1980
Palmer RN, Rick ME, Rick PD, Zeller JA, Gralnick HR: Circulating heparan sulfate anticoagulant in a patient with a fatal bleeding disorder. N Engl J Med 310: 1696–1699, 1984
Russel JB, Steinhertz PG, Miller DR, Hilgartner MW: A heparin-like anticoagulant in an 8-month-old boy with acute monoblastic leukemia. Am J Hematol 16: 83–90, 1984
Marcum JA, Atha DH, Fritze LMS, Nawroth P, Stern D, Rosenberg RD: Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem 261: 7507–7517, 1986
Cines DB, Tomaski A, Tannenbaum S: Immune endothelial-cell injury in heparin-associated thrombocytopenia. N Engl J Med 316: 581–589, 1987
Kramer RH, Vogel KG, Nicolson GL: Solubilization and degradation of subendothelial matrix glycoproteins and proteoglycans by metastatic tumor cells. J Biol Chem 257: 2678–2686, 1982
Kramer RH, Vogel KG: Selective degradation of basement membrane macromolecules by metastatic melanoma cells. J Natl Cancer Inst 72: 889–899, 1984
Nakajima M, Irimura T, Di Ferrante D, Di Ferrante N, Nicolson GL: Heparan sulfate degradation: Relation to tumor invasive and metastatic properties of mouse B16 melanoma sublines. Science 220: 611–613, 1983
Nakajima M, Irimura T, Di Ferrante ND, Nicolson GL: Metastatic melanoma cell heparanase. Characterization of heparan sulfate degradation fragments produced by B16 melanoma endoglucuronidase. J Biol Chem 259: 2283–2290, 1984
Vlodavsky I, Fuks Z, Bar-Ner M, Ariav Y, Shirrmacher V: Lymphoma cell-mediated degradation of sulfated proteoglycans in the subendothelial extracellular matrix: relationship to tumor cell metastasis. Cancer Res 43: 2704–2711, 1983
Iozzo RV: Biosynthesis of heparan sulfate proteoglycan by human colon carcinoma cells and its localization at the cell surface. J Cell Biol 99: 403–417, 1984
Iozzo RV, Ketterer CL, Slaymaker DJ: Evidence of a small hydrophobic domain in the core protein of heparan sulfate proteoglycan from human colon carcinoma cells. FEBS Lett 206: 304–308, 1986
Iozzo RV: Turnover of heparan sulfate proteoglycan in human colon carcinoma cells. A quantitative biochemical and autoradiographic study. J Biol Chem 262: 1888–1900, 1987
Kjellén L, Pertoft H, Oldberg Å, Höök M: Oligosaccharides generated by an endoglueuronidase are intermediates in the intracellular degradation of heparan sulfate proteoglycans. J Biol Chem 260: 8416–8422, 1985
Liotta LA, Rao CN, Barsky SH: Tumor invasion and the extracellular matrix. Lab Invest 49: 636–649, 1983
Woolley DE: Collagenolytic mechanisms in tumor cell invasion. Cancer Metastasis Rev 3: 361–372, 1984
Wewer UM, Albrechtsen R, Rao CN, Liotta LA: The extracellular matrix in malignancy. In: Kuhn K (ed) Rheumatology. S. Karger, Basel, 1986, pp 451–478
Iozzo RV: Neoplastic modulation of extracellular matrix. Colon carcinoma cells release polypeptides that alter proteoglycan metabolism in colon fibroblasts. J Biol Chem 260: 7464–7473, 1985
Knudson W, Biswas C, Toole BP: Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci USA 81: 6767–6771, 1984
Merrilees MJ, Finlay GJ: Human tumor cells in culture stimulate glycosaminoglycan synthesis by human skin fibroblasts. Lab Invest 53: 30–36, 1985
Iozzo RV, Muller-Glauser W: Neoplastic modulation of extracellular matrix: proteoglycan changes in the rabbit mesentery induced by V2 carcinoma cells. Cancer Res 45: 5677–5687, 1985
Dvorak HF: Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659, 1987
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iozzo, R.V. Proteoglycans and neoplasia. Cancer Metast Rev 7, 39–50 (1988). https://doi.org/10.1007/BF00048277
Issue Date:
DOI: https://doi.org/10.1007/BF00048277