Abstract
The use of fossil fuel is predicted to cause an increase of the atmospheric CO2 concentration, which will affect the global pattern of temperature and precipitation. It is therefore essential to incorporate effects of temperature and water supply on the carbon requirement for root respiration of plants to predict effects of elevated [CO2] on the carbon budget of natural and managed systems.
There is insufficient information to support the contentention that an increase in the concentration of CO2 in the atmosphere will enhance the CO2 concentration in the soil to an extent that is likely to affect root respiration. Moreover, there is no convincing evidence for a direct effect of elevated atmospheric [CO2] on the rate of root respiration per unit root mass or the fraction of carbon required for root respiration. However, there are likely to be indirect effects of elevated [CO2] on the carbon requirement of plants in natural systems.
Firstly, it is very likely that the carbon requirement of root respiration relative to that fixed in photosynthesis will increase when elevated [CO2] induces a decrease in nutrient status of the plants. Although earlier papers have emphasized that elevated [CO2] favours investment of biomass in roots relative to that in leaves, these are in fact indirect effects. The increase in root weight ratio is due to the more rapid depletion of nutrients in the root environment as a consequence of enhanced growth. This will decrease the specific rate of root respiration, but increase the carbon requirement as a fraction of the carbon fixed in photosynthesis. It is likely that these effects will be minor in systems where the nutrient supply is very high, e.g. in many managed arable systems, and increase with decreasing soil fertility, i.e. in many natural systems.
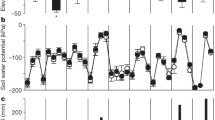
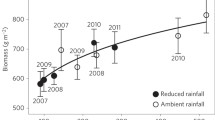
Secondly, a decrease in rainfall in some parts of the world may cause a shortage in water supply which favours the carbon partitioning to roots. Water stress is likely to reduce rates of root respiration per unit root mass, but enhance the fraction of total assimilates required for root respiration, due to greater allocation of biomass to roots.
Increased temperatures are unlikely to affect the specific rate of root respiration in all species. Broadly generalized, the effect of temperature on biomass allocation is that the relative investment of biomass in roots is lowest at a certain optimum temperature and increases at both higher and lower temperatures. The root respiration of some species acclimates to growth temperature, so that the effect of global temperature rise is entirely accounted for by the effect of temperature on biomass allocation. The specific rate of root respiration of other species will increase with global warming. In response to global warming the carbon requirement of roots is likely to decrease in temperate regions, when temperatures are suboptimal for the roots' capacity to acquire water. Here global warming will induce a smaller biomass allocation to the roots. Conversely, the carbon requirements are more likely to increase in mediterranean environments, where temperatures are often supraoptimal and a rise in temperature will induce greater allocation of biomass to the roots.
Similar content being viewed by others
References
Amthor, J S, Koch, G W and Bloom, A J 1992 CO2 inhibits respiration in leaves of Rumex crispus L. Plant Physiol. 98, 757–760.
Atkin, O K, Villar, R and Lambers, H 1995 Partitioning of electrons between the cytochrome and the alternative pathways in intact roots. Plant Physiol. 108, 1179–1183.
Atkin O K, Botman B and Lambers H 1996 The causes of inherently slow growth in alpine plants: an analysis based on the underlying carbon economies of alpine and lowland Poa species. Funct. Ecol. (In press).
Azcón-Bieto, J, Gonzalez-Mehler, M A, Doherty, W and Drake, B G 1992 Acclimation of respiratory properties O2 uptake in green tissues of filed-grown native species after long-term exposure to elevated atmospheric CO2. Plant Physiol. 106, 1163–1168
Baas, R, Van derWerf, A and Lambers, H 1989 Analysis of growth and root respiration in Plantago major ssp. pleiosperma: effects of VA mycorrhizal infection and P addition. Plant Physiol. 91, 227–232.
Bakker, J P 1989 Nature Management by Grazing and Cutting. Kluwer Academic Publishing, Dordrecht, the Netherlands.
Blacquière, T and Lambers, H 1981 Growth, photosynthesis and respiration in Plantago coronopus as affected by salinity. Physiol. Plant. 51, 265–268.
Bowen, G D 1991 Soil temperature, root growth, and plant function. In Plant Roots: The Hidden Half. Eds. YWaisel, AEshel and UKafkaki. pp 309–330. Plenum Press, New York, USA.
Brouwer, R 1963 Some aspects of the equilibrium between overground and underground plant parts. Meded. Inst. Biol. Scheikd. Onderz. Landbouwgew. 213, 31–39.
Brouwer, R 1983 Functional equilibrium: sense or nonsense? Neth. J. Agric. Sci. 31, 305–311.
Burchett, M D, Field, C D and Pulkownik, A 1984 Salinity, growth and root respiration in the grey mangrove, Avicennia marina. Physiol. Plant. 60, 113–118.
Chu, C C, Coleman, J S and Mooney, H A 1992 Controls of biomass partitioning between roots and shoots: Atmospheric CO2 enrichment and the acquisition and allocation of carbon and nitrogen in wild radish. Plant Cell Environ. 89, 580–587.
Creelman, R A, Mason, H S, Bensen, R J, Boyer, J S and Mullet, J E 1990 Water deficit and abscisic acid cause differential inhibition of shoot versus root growth in soybean seedlings. Analysis of growth, sugar accumulation, and gene expression. Plant Physiol. 92, 205–214.
Curtis, S, O'Neil, E G, Teeri, J A, Zak, D R and Pregitzer, K S 1994 Belowground responses to rising atmospheric CO2: Implications for plants, soil biota and ecosystem processes. Plant and Soil 165, 1–6.
Davies, W and Zhang, J 1991 Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Molec. Biol. 42, 55–76.
Davies, W J, Tardieu, F and Trejo, C L 1994 How do chemical signals work in plants that grow in drying soil? Plant Physiol. 104, 309–314.
Day, D A, Krab, K, Lambers, H, Moore, A L, Siedow, J N, Wagner, A M and Wiskich, J T 1996 The cyanide-resistant oxidase: to inhibit or nut to inhibit, that is the question. Plant Physiol. 110, 1–2.
DenHertog, J, Stulen, I and Lambers, H 1993 Assimilation, respiration and allocation of carbon in Plantago lanceolata as affected by atmospheric CO2. Vegetatio 104/105, 369–378.
Den Hertog J, Stulen I, Fonseca F and Delea P 1996 Modulation of carbon and nitrogen allocation in Urtica dioica and Plantago major by elevated CO2: Impact of accumulation of nonstructural carbohydrates and ontogenetic drift. Physiol. Plant. (In press).
Elberse, W Th and Berendse, F 1993 A comparative study of the drowth and morphology of eight grass species from habitats with different nutrient availabilities. Funct. Ecol. 7, 223–229.
El Kohen A, Pontailler J-Y and Mousseau M 1991 Effect of doubling of atmospheric CO2 concentration on dark respiration in aerial parts of young chestnut trees (Castanea sativa Mill.). C.R. Acad. Sci, Paris, t. 312, Series III, 477–481.
Farrar, J F 1992 The whole plant: carbon partitioning during development. In Carbon partitioning within and between organs. Eds. C JPollock, J FFarrar and A JGordon. pp 163–180. Bios Scientific Publishers Ltd., Oxford, UK.
Farrar, J F and Williams, M L 1991 The effects of increased atmospheric carbon dioxide on carbon partitioning, source-sink relations and respiration. Plant Cell Environ. 14, 819–830.
Fonseca F G 1996 The response of herbaceous plants to elevated C2. Short term modification of C and N partitioning and growth of Plantago major ssp. pleiosperma. PhD Thesis, University of Groningen, Groningen, the Netherlands.
Fonseca F G, Den Hertog J and Stulen I 1996 The response of Plantago major ssp. pleiosperma to elevated CO2 is modulated by the formation of secondary shoots. New Phytol. (In press).
Foth, H D 1962 Root and top growth of corn. Agron. J. 54, 49–52.
Gifford, R M, Lambers, H and Morison, J I L 1985 Respiration of crop plants under CO2 enrichment. Physiol. Plant. 63, 351–356.
Good, B J and Patrick, W H 1987 Gas composition and respiration of water oak (Quercus nigra L.) and green ash (Fraxinus pennsylvanica Marsh.) roots after prolonged flooding. Plant and Soil 97, 419–427.
Hoefnagel, M H N, Millar, A H, Wiskich, J T and Day, J T 1995 Cytochrome and alternative respiratory pathways compete for electrons in the presence of pyruvate in soybean mitochondria. Arch. Biochem. Biophys. 318, 394–400.
Houghton, J T, Callander, B A and Varney, S K 1992 Climate change 1992: the supplementary report to the IPCC scientific assessment. Cambridge University Press, Cambridge, UK.
Körner, C and Larcher, W 1988 Plant life in cold environments. In Plants and Temperature, Symp. Soc. Exp. Biol. Vol. 42. Eds. S FLong and F IWoodward. pp 25–57. The Company of Biologists Limited, Cambridge, UK.
Kuiper, D 1983 Genetic differentiation in Plantago major Growth and root respiration and their role in phenotypic adaptation. Physiol. Plant. 57, 222–230.
Lambers, H 1983 The ‘functional equilibrium’: Nibbling on the edges of a paradigm. Neth. J. Agric. Sci. 31, 305–311.
Lambers, H and Atkin, OK 1995 Regulation of carbon metabolism in roots. In Carbon Partitioning and Source-Sink Interactions in Plants. Eds. M AMadore and W JLucas. pp 226–238. American Socie of Plant Physiologists, Rockville, USA.
Lambers, H, Blacquière, T and Stuiver, C E E 1981 Interactions between osmoregulation and the alternative respiratory pathway in Plantago coronopus as affected by salinity. Physiol. Plant. 51, 63–68.
Lambers, H, Szaniawski, R K and DeVisser, R 1983 Respiration for growth, maintenance and ion uptake. An evaluation of concept, methods, values and their significance. Physiol. Plant. 58, 556–563.
Lambers, H, Van denBoogaard, R, Veneklaas, E J and Villar, R 1995 Effects of global environmental change on carbon partitioning in vegetative plants of Triticum aestivum and closely related Aegilops species. Global Change Biol. 1, 397–406.
Lambers, H, Atkin, O K and Scheurwater, I 1996 Respiratory patterns in roots in relation to their functioning. In Plant Roots: The Hidden Half. Eds. YWaisel, AEshel and UKafkaki. pp 323–362. Marcel Decker, Inc., New York, USA.
Larigauderie, A, Reynolds, J F and Strain, B R 1994 Root responses to CO2 enrichment and nitrogen supply in loblolly pine. Plant and Soil 165, 21–32.
Li, X, Feng, Y and Boersma, L 1994 Partitioning of photosynthates between shoot and root in spring wheat (Triticum aestivum L.) as a function of soil water potential and root temperature. Plant and Soil 164, 43–50.
Luo, Y, Field, C B and Mooney, H A 1994 Predicting responses of photosynthesis and root fraction to elevated [CO2] a : interactions among carbon, nitrogen, and growth. Plant Cell Environ. 17, 1195–1204.
McKee, I F and Woodward, F I 1994 CO2 enrichment responses of wheat: interactions with temperature, nitrate and phosphate. New Phytol. 127, 447–453.
Millar, A H, Atkin, O K, Lambers, H, Wiskich, J T and Day, D A, 1995 A critique of the use of inhibitors to estimate partitioning of electrons between mitochondrial respiratory pathways in plants. Physiol. Plant. 95, 523–532.
Newberry, R M, Wolfenden, J, Mansfield, T A and Harrison, A F 1995 Nitrogen, phosphorus and potassium uptake and demand in Agrostis capillaris: the influence of elevated CO2 and nutrient supply. New Phytol. 130, 565–574.
Nicolas, M E, Lambers, H, Simpson, R J and Dalling, M J 1985 Effect of drought on metabolism and partitioning of carbon in two varieties of wheat differing in drought tolerance. Ann. Bot. 55, 727–742.
Nobel, P S and Palta, J A 1989 Soil O2 and CO2 effects on root respiration of cacti. Plant and Soil 120, 263–271.
Norby, R J 1994 Issues and perspectives for investigating root responses to elevated atmospheric carbon dioxide. Plant and Soil 165, 9–20.
Norby, R J and O'Neill, E 1991 Leaf area compensation and nutrient interactions in CO2-enriched seedlings of yellow poplar (Liriodendron tulipifera L.). New Phytol. 117, 515–528.
Norby, R J, O'Neill, E G, Hood, W G and Luxmoore, R J 1987 Carbon allocation, root exudation, and mycorrhizal colonization of Pinus echinata seedlings grown under CO2 enrichment. Tree Physiol. 3, 203–210.
Olff, H and Bakker, J P 1991 Long-term dynamics of standing crop and species composition after the cessation of fertilizer application to mown grassland. J. Appl. Ecol. 28, 1040–1052.
Olff, H, Berendse, F and DeVisser, W 1994 Changes in mineralization, tissue nutrient concentrations and biomass compartmentation after cessation of fertilizer application to mown grassland. J. Ecol. 82, 611–620.
Palet, A, Ribas-Carbó, M, Argiles, J M and Azcòn-Bieto, J 1991 Short-term effects of carbon dioxide on carnation callus cell respiration. Plant Physiol. 96, 467–472.
Palet, A, Ribas-Carbó, M, Gonzalez-Meler, A, Aranda, X and Azcòn-Bieto, J 1992 Short-term effects of CO2/bicarbonate on plant cell respiration. In Molecular Biochemical and Physiological Aspects of Plant Respiration. Eds. HLambers and L H WVan derPlas. pp 597–601. SPB Academic Publishing, The Hague, the Netherlands.
Palta, J A and Nobel, P S 1989 Influence of soil O2 and CO2 on root respiration for Agave deserti. Physiol. Plant. 76, 187–192.
Pate, J S, Layzell, D B and Atkins, C A 1979 Economy of carbon and nitrogen in a nodulated and nonnodulated (NO3-grown) legume. Plant Physiol. 64, 1083–1088.
Poorter, H, Pot, S and Lambers, H 1988 The effect of an elevated atmospheric CO2 concentration on growth, photosynthesis and respiration of Plantago major. Physiol. Plant. 73, 553–559.
Poorter, H, Remkes, C and Lambers, H 1990 Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol. 94, 621–627.
Poorter, H, Gifford, R M, Kriedemann, P E and Wong, S C 1992 A quantitative analysis of dark respiration and carbon content as factors in the growth response of plants to elevated CO2. Aust. J. Plant Physiol. 40, 501–513.
Poorter, H, Van deVijver, C A D M, Boot, R G A and Lambers, H 1995 Growth and carbon economy of a fast-growing and a slow-growing grass species as dependent on nitrate supply. Plant and Soil 171, 217–227.
Qi, J, Marshall, J D and Mattson, K G 1994 High soil carbon dioxide concentrations inhibit root respiration of Douglas fir. New Phytol. 128, 435–442.
Rychter, A M and Mikulska, M 1990 The relationship between phosphate status and cyanide-resistant respiration in bean roots. Physiol. Plant. 79, 663–667.
Saab, I N, Sharp, R E, Pritchard, J and Voetberg, G S 1990 Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol. 93, 1329–1336.
Sharp, R E and Davies, W J 1979 Solute regulation and growth by roots and shoots of water-stressed maize plants. Planta 147, 43–49.
Smakman, H and Hofstra, R 1982 Energy metabolism of Plantago lanceolata as affected by change in root temperature. Physiol. Plant. 56, 33–37.
Sowell, J B and Spomer, G G 1986 Ecotypic variation in root respiration rate among elevational populations of Abies lasiocarpa and Picea engelmannii. Oecologia 68, 375–379.
Stulen, I and DenHertog, J 1993 Root growth and functioning under atmospheric CO2 enrichment. Vegetatio 104/105, 99–115.
Stulen, I, DenHertog, J Drelon, F and Roy, J 1994 An integrated approach to the influence of CO2 on plant growth using data for three herbaceous species. In A whole Plant Perspective on Carbon-Nitrogen Interactions. Eds. JRoy and EGarnier. pp 229–246. SPB Academic Publishing, The Hague, the Netherlands.
Szaniawski, R K 1983 Adaptation and functional balance between shoot and root activity of sunflower plants grown at different root temperature. Ann. Bot. 51, 453–459.
Szaniawski, R K and Kielkiewicz, M 1982 Maintenance and growth respiration in shoots and roots of sunflower plants grown at different root temperatures. Physiol. Plant. 54, 500–504.
Van derWerf, A 1996 Growth analysis and photoassimilate partitioning. In Photoassimilate distribution in Plants and Crops: Source-sink Relationships. Eds. EZamski and A ASchaffer. pp 1–20. Marcel Dekker, New York, USA.
Van der Werf A and Nagel O W 1996 Carbon allocation to shoots and roots in relation to nitrogen supply is mediated by cytokinins and sucrose: opinion. Plant and Soil (In press).
Van derWerf, A, Welschen, R and Lambers, H 1992 Respiratory losses increase with decreasing inherent growth rate of a species and with decreasing nitrate supply: a search for explanations for these observations. In Molecular, Biochemical and Physiological Aspects of Plant Respiration. Eds. HLambers and L H WVan derPlas. pp 421–432. SPB Academic Publishing, TheHague, the Netherlands.
Van derWerf, A, VanNuenen, M, Visser, A J and Lambers, H 1993a Effects of N-supply on the rate of photosynthesis and shoot and respiration of inherently fast- and slow-growing monocotyledonous species. Physiol. Plant. 89, 563–569.
Van derWerf, A, Visser, A J, Schieving, F and Lambers, H 1993b Evidence for optimal partitioning of biomass and nitrogen at a range of nitrogen availabilities for a fast- and slow-growing species. Funct. Ecol. 7, 63–74.
Van derWerf, A, Enserink, T, Smit, B and Booij, R 1993c Allocation of carbon and nitrogen as a function of the internal nitrogen status of a plant: Modelling allocation under non-steady-state conditions. Plant and Soil 155/156, 183–186.
Van derWerf, A, Poorter, H and Lambers, H 1994 Respiration as dependent on a species's inherent growth rate and on the nitrogen supply to the plant. In A Whole-Plant Perspective of Carbon-Nitrogen Interactions. Eds. JRoy and EGarnier. pp 61–77. SPB Academic Publishing, The Hague, the Netherlands.
VanKeulen, H and Seligman, N G 1987 Simulation of Water Use, Nitrogen Nutrition and Growth of a Spring Wheat Crop. Pudoc, Wageningen, the Netherlands.
Veen, B W 1980 Energy costs of ion transport. In Genetic Engineering of Osmoregulation. Impact on Plant Productivity for Food, Chemicals and Energy. Eds. D WRains, R CValentine and A Hollaender. pp 187–195. Plenum Press, New York, USA.
Vivin, P, Gross, P, Aussenac, G and Guehl, J-M 1995 Whole plant CO2, exchange, carbon partitioning and growth on Quercus robur seedlings exposed to elevated CO2. Plant Physiol. Biochem. 33, 201–211.
Walters, M B, Kruger, E L and Reich, P B 1993 Relative growth rate in relation to physiological traits for northern hardwood tree seedlings: species, light environment and ontogenetic considerations. Oecologia 9, 219–231.
Whitehead, D C 1986 Sources and transformations of organic nitrogen in intensively managed grassland soils. In Nitrogen Fluxes in Intensive Grassland Systems. Eds. H GVan derMeer, J CRyden and G CEnnik. pp 47–58. Martinus Nijhoff Publishers, Dordrecht, the Netherlands.
Weger, H G and Guy, R D 1991 Cytochrome and alternative pathway respiration in white spruce (Picea glauca) roots. Effects of growth and measurement temperature. Physiol. Plant. 83, 675–681.
Williams, J H H, Winters, A L and Farrar, J F 1992 Sucrose: a novel plant growth regulator. In Plant Respiration. Molecular, Biochemical and Physiological Aspects. Eds. HLambers and L H WVan derPlas. pp 463–469. SPB Academic Publishing, The Hague, the Netherlands.
Wong, S C 1979 Elevated atmospheric partial pressure of CO2 and plant growth. I. Interactions of nitrogen nutrition and photosynthetic capacity in C3 and C4 plants. Oecologia 44, 68–74.
Zimmerman, R C, Smith, R D and Alberte, R S 1989 Thermal acclimation and whole-plant carbon balance in Zostera marina L. (eelgrass). J. Exp. Mar. Biol. Ecol. 130, 93–109.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lambers, H., Stulen, I. & van der Wert, A. Carbon use in root respiration as affected by elevated atmospheric O2 . Plant Soil 187, 251–263 (1995). https://doi.org/10.1007/BF00017091
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00017091