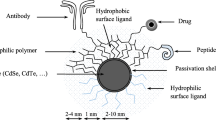
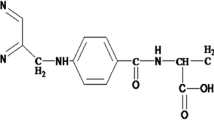
Abstract
Cancer, being a condition of unhindered cell growth ultimately leading to the death of the patient, is very prominent in modern society. Despite all the colloquial therapies to stop or prevent or cure cancer, it’s not curable to a full extent. These colloquial therapies used in the treatment of cancer involve chemotherapy, radiation therapy, etc., leading to very prominent side effects and more often than not, the recurrence of the disease. Thus, the need for an alternative to the treatment of cancer is of utmost and urgent requirement; in lieu of this fact, the quantum dots, which are semiconductor nanocrystals in the range of 1–10 nm are employed as a complete theranostic tool for the same. Recent progress in the quantum dots technology allows us to both image and kill cancer cells with high target specificity and minimum or no side effects. Various organic, as well as inorganic quantum dots, are being employed to cure cancer as it is or in conjugation with various biomarkers like peptides, transferrin, folic acid, etc. to increase target specificity and minimize side effects. Though a lot of research review, have been written over the past few years in the last decade, in this review we will be focusing on certain types of cancers and the treatments generally employed and their drawbacks, and the development with the current status of the quantum dots technology in the treatment of cancer and its drawbacks like cytotoxicity.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Sanz-Moreno V, Marshall CJ. The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr Opin Cell Biol. 2010;22(5):690–6.
Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: Integrating signals from front to back. Science. 2003;302(5651):1704–9.
Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84(3):359–69.
Friedl P, Alexander S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell. 2011;147(5):992–1009.
Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22(5):697–706.
Alexander S, Friedl P. Cancer invasion and resistance: Interconnected processes of disease progression and therapy failure. Trends Mol Med. 2012;18(1):13–26.
Yao H, Zeng ZZ, Fay KS, Veine DM, Staszewski ED, Morgan M, et al. Role of α(5)β(1) integrin up-regulation in radiation-induced invasion by human pancreatic cancer cells. Transl Oncol. 2011;4(5):282–92.
Nelson CM, Khauv D, Bissell MJ, Radisky DC. Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells. J Cell Biochem. 2008;105(1):25–33.
Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: Dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28(1–2):167–76.
Kim D, Yoo JM, Hwang H, Lee J, Lee SH, Yun SP, et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat Nanotechnol. 2018;13(9):812–8.
Chung YJ, Kim K, Lee BI, Park CB. Carbon nanodot-sensitized modulation of Alzheimer’s β-amyloid self-assembly. Disassembly Toxicity. 2017;13(34):1700983.
Bentolila LA, Ebenstein Y, Weiss S. Quantum dots for in vivo small-animal imaging. J Nucl Med Official Publ Soc Nucl Med. 2009;50(4):493–6.
Hilderbrand SA, Weissleder R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr Opin Chem Biol. 2010;14(1):71–9.
Wang Y, Chen L. Quantum dots, lighting up the research and development of nanomedicine. Nanomedicine. 2011;7(4):385–402.
Tholouli E, Sweeney E, Barrow E, Clay V, Hoyland JA, Byers RJ. Quantum dots light up pathology. J Pathol. 2008;216(3):275–85.
Byers RJ, Hitchman ER. Quantum dots brighten biological imaging. Prog Histochem Cytochem. 2011;45(4):201–37.
True LD, Gao X. Quantum dots for molecular pathology: Their time has arrived. J Mol Diagn. 2007;9(1):7–11.
He X, Gao J, Gambhir SS, Cheng Z. Near-infrared fluorescent nanoprobes for cancer molecular imaging: Status and challenges. Trends Mol Med. 2010;16(12):574–83.
Fang M, Peng CW, Pang DW, Li Y. Quantum dots for cancer research: Current status, remaining issues, and future perspectives. Cancer Biol Med. 2012;9(3):151–63.
Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13(3):372–7.
Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc. 2004;126(40):12736–7.
Li S, Skromne I, Peng Z, Dallman J, Al-Youbi AO, Bashammakh AS, et al. “Dark” carbon dots specifically “light-up” calcified zebrafish bones. J Mater Chem B. 2016;4(46):7398–405.
Peng Z, Miyanji EH, Zhou Y, Pardo J, Hettiarachchi SD, Li S, et al. Carbon dots: promising biomaterials for bone-specific imaging and drug delivery. Nanoscale. 2017;9(44):17533–43.
Gao X, Du C, Zhuang Z, Chen W. Carbon quantum dot-based nanoprobes for metal ion detection. J Mater Chem C. 2016;4(29):6927–45.
Margraf JT, Strauss V, Guldi DM, Clark T. The electronic structure of amorphous carbon nanodots. J Phys Chem B. 2015;119(24):7258–65.
Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Controlled Release: Official J Controlled Release Soc. 2018;270:290–303.
Li S, Amat D, Peng Z, Vanni S, Raskin S, De Angulo G, et al. Transferrin conjugated nontoxic carbon dots for doxorubicin delivery to target pediatric brain tumor cells. Nanoscale. 2016;8(37):16662–9.
Yu B, Tai HC, Xue W, Lee LJ, Lee RJ. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol Membr Biol. 2010;27(7):286–98.
Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166(20):2244–52.
Mustacchi G, De Laurentiis M. The role of taxanes in triple-negative breast cancer: literature review. Drug Des Devel Ther. 2015;9:4303–18.
Åkerman ME, Chan WCW, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc Natl Acad Sci. 2002;99(20):12617.
Ruan J, Wang K, Song H, Xu X, Ji J, Cui D. Biocompatibility of hydrophilic silica-coated CdTe quantum dots and magnetic nanoparticles. Nanoscale Res Lett. 2011;6(1):299.
Cai X, Luo Y, Zhang W, Du D, Lin Y. pH-sensitive ZnO quantum dots-doxorubicin nanoparticles for lung cancer targeted drug delivery. ACS Appl Mater Interfaces. 2016;8(34):22442–50.
Li S, Yang J, Lei X, Zhang J, Yang H, Li K, et al. Peptide-conjugated quantum dots act as the target marker for human pancreatic carcinoma cells. Cell Physiol Biochem. 2016;38(3):1121–8.
Selvan ST, Patra PK, Ang CY, Ying JY. Synthesis of silica-coated semiconductor and magnetic quantum dots and their use in the imaging of live cells. Angew Chem. 2007;119(14):2500–4.
Yang S-T, Cao L, Luo PG, Lu F, Wang X, Wang H, et al. Carbon dots for optical imaging in vivo. J Am Chem Soc. 2009;131(32):11308–9.
Zhang X, Meng L, Lu Q, Fei Z, Dyson PJ. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials. 2009;30(30):6041–7.
Singh RP, Sharma G, Sonali, Singh S, Patne SCU, Pandey BL, et al. Effects of transferrin conjugated multi-walled carbon nanotubes in lung cancer delivery. Mater Sci Eng C. 2016;67:313–25.
Zhao X, Zhang J, Shi L, Xian M, Dong C, Shuang S. Folic acid-conjugated carbon dots as green fluorescent probes based on cellular targeting imaging for recognizing cancer cells. RSC Adv. 2017;7(67):42159–67.
Mewada A, Pandey S, Thakur M, Jadhav D, Sharon M. Swarming carbon dots for folic acid mediated delivery of doxorubicin and biological imaging. J Mater Chem B. 2014;2(6):698–705.
Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469–87.
Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21(17):3357–65.
Leong ASY, Zhuang Z. The changing role of pathology in breast cancer diagnosis and treatment. Pathobiology. 2011;78(2):99–114.
Decker T, Hungermann D, Böcker W. Prognostic and predictive factors of invasive breast cancer: Update 2009. Pathologe. 2009;30(1):49–55.
Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485–91.
Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118–28.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108.
Li D, Kang J, Golas BJ, Yeung VW, Madoff DC. Minimally invasive local therapies for liver cancer. Cancer Biol Med. 2014;11(4):217–36.
Goulart BH, Bensink ME, Mummy DG, Ramsey SD. Lung cancer screening with low-dose computed tomography: costs, national expenditures, and cost-effectiveness. J Nat Compr Cancer Netw JNCCN. 2012;10(2):267–75.
Al BB, Tanios B-S, Emily C, Yi-Jen C, Michael AC, Harry SC, et al. Metastatic colon cancer, version 3.2013. J Nat Compr Cancer Netw. 2013;11(2):141–52.
Benson A, Abrams T, Ben-Josef E, Bloomston M, Clary B, Covey A, et al. Hepatobiliary cancers. J Nat Compr Cancer Netw JNCCN. 2009;7.
Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: Response-based selection and complete resection define outcome. J Clin Oncol. 2011;29(8):1083–90.
<Local Therapies for Hepatic Metastases.pdf>.
Hohenberger P, Gretschel S. Gastic cancer. The Lancet. 2003;362(9380):305–15.
Parkin DM. International variation. Oncogene. 2004;23(38):6329–40.
Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer (Oxford, England: 1990). 2001;37 Suppl 8:S4–66.
Huang P, Xu C, Lin J, Wang C, Wang X, Zhang C, et al. Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics. 2011;1:240–50.
Huang P, Bao L, Zhang C, Lin J, Luo T, Yang D, et al. Folic acid-conjugated silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomaterials. 2011;32(36):9796–809.
Tian B, Wang C, Zhang S, Feng L, Liu Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano. 2011;5(9):7000–9.
Ma LL, Feldman MD, Tam JM, Paranjape AS, Cheruku KK, Larson TA, et al. Small multifunctional nanoclusters (Nanoroses) for targeted cellular imaging and therapy. ACS Nano. 2009;3(9):2686–96.
He M, Huang P, Zhang C, Hu H, Bao C, Gao G, et al. Dual phase-controlled synthesis of uniform lanthanide-doped NaGdF4 upconversion nanocrystals via an OA/ionic liquid two-phase system for in vivo dual-modality imaging. Adv Func Mater. 2011;21(23):4470–7.
Lee EC, Yang JY, Lee KG, Oh SY, Suh YS, Kong SH, et al. The value of postoperative serum carcinoembryonic antigen and carbohydrate antigen 19–9 levels for the early detection of gastric cancer recurrence after curative resection. J Gastric Cancer. 2014;14(4):221–8.
Asao T, Fukuda T, Yazawa S, Nagamachi Y. Carcinoembryonic antigen levels in peritoneal washings can predict peritoneal recurrence after curative resection of gastric cancer. Cancer. 1991;68(1):44–7.
Marrelli D, Pinto E, De Stefano A, Farnetani M, Garosi L, Roviello F. Clinical utility of CEA, CA 19–9, and CA 72–4 in the follow-up of patients with respectable gastric cancer. Am J Surg. 2001;181(1):16–9.
Duan D, Fan K, Zhang D, Tan S, Liang M, Liu Y, et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens Bioelectron. 2015;74:134–41.
Sudjaroen Y. Efficiency assessment of immunochromatographic strip test for the diagnosis of alpha-thalassemia-1 carriers. J Lab Physicians. 2015;7(1):4–10.
Vyas SS, Jadhav SV, Majee SB, Shastri JS, Patravale VB. Development of immunochromatographic strip test using fluorescent, micellar silica nanosensors for rapid detection of B. abortus antibodies in milk samples. Biosens Bioelectron. 2015;70:254–60.
Yan X, Wang K, Lu W, Qin W, Cui D, He J. CdSe/ZnS quantum dot-labeled lateral flow strips for rapid and quantitative detection of gastric cancer carbohydrate antigen 72–4. Nanoscale Res Lett. 2016;11(1):138.
Soltesz EG, Kim S, Kim SW, Laurence RG, De Grand AM, Parungo CP, et al. Sentinel lymph node mapping of the gastrointestinal tract by using invisible light. Ann Surg Oncol. 2006;13(3):386–96.
Parungo CP, Ohnishi S, Kim SW, Kim S, Laurence RG, Soltesz EG, et al. Intraoperative identification of esophageal sentinel lymph nodes with near-infrared fluorescence imaging. J Thorac Cardiovasc Surg. 2005;129(4):844–50.
Ruan J, Song H, Qian Q, Li C, Wang K, Bao C, et al. HER2 monoclonal antibody conjugated RNase-A-associated CdTe quantum dots for targeted imaging and therapy of gastric cancer. Biomaterials. 2012;33(29):7093–102.
Kong Y, Chen J, Gao F, Li W, Xu X, Pandoli O, et al. A multifunctional ribonuclease-A-conjugated CdTe quantum dot cluster nanosystem for synchronous cancer imaging and therapy. Small. 2010;6(21):2367–73.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Wong MCS, Lao XQ, Ho KF, Goggins WB, Tse SLA. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep. 2017;7(1):14300.
Bjerager M, Palshof T, Dahl R, Vedsted P, Olesen F. Delay in diagnosis of lung cancer in general practice. Br J Gen Pract. 2006;56(532):863–8.
Singh RD, Shandilya R, Bhargava A, Kumar R, Tiwari R, Chaudhury K, et al. Quantum dot based nano-biosensors for detection of circulating cell free mirnas in lung carcinogenesis: From biology to clinical translation. Front Genet. 2018;9:616.
Reduced lung-cancer mortality with low-dose computed tomographic screening. New England J Med. 2011;365(5):395–409.
Wildstein KA, Faustini Y, Yip R, Henschke CI, Ostroff JS. Longitudinal predictors of adherence to annual follow-up in a lung cancer screening programme. J Med Screen. 2011;18(3):154–9.
Brenner DJ. Radiation and chest CT scans: Are there problems? What should we do? Chest. 2012;142(3):549–50.
McCunney RJ, Li J. Radiation risks in lung cancer screening programs. Chest. 2014;145(3):618–24.
Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2012;64:24–36.
Schroeder KL, Goreham RV, Nann T. Graphene quantum dots for theranostics and bioimaging. Pharm Res. 2016;33(10):2337–57.
Alessandro A, Giuseppa P, Andrea A, Vincenzo R, Sabina R, Caterina M. Nanoparticles in oncology: The new theragnostic molecules. Anticancer Agents Med Chem. 2011;11(7):669–86.
Mariappan L, Shao Q, Jiang C, Yu K, Ashkenazi S, Bischof JC, et al. Magneto acoustic tomography with short pulsed magnetic field for in-vivo imaging of magnetic iron oxide nanoparticles. Nanomedicine. 2016;12(3):689–99.
FitzGerald PF, Butts MD, Roberts JC, Colborn RE, Torres AS, Lee BD, et al. A proposed computed tomography contrast agent using carboxybetaine Zwitterionic tantalum oxide nanoparticles: Imaging, biological, and physicochemical performance. Invest Radiol. 2016;51(12):786–96.
Lu A-H, Zhang X-Q, Sun Q, Zhang Y, Song Q, Schüth F, et al. Precise synthesis of discrete and dispersible carbon-protected magnetic nanoparticles for efficient magnetic resonance imaging and photothermal therapy. Nano Res. 2016;9(5):1460–9.
Stone RC, Fellows BD, Qi B, Trebatoski D, Jenkins B, Raval Y, et al. Highly stable multi-anchored magnetic nanoparticles for optical imaging within biofilms. J Colloid Interface Sci. 2015;459:175–82.
Zhou Q, Wei Y. for better or worse, iron overload by superparamagnetic iron oxide nanoparticles as a MRI contrast agent for chronic liver diseases. Chem Res Toxicol. 2017;30(1):73–80.
Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307(5709):538–44.
Alivisatos AP, Gu W, Larabell C. Quantum dots as cellular probes. Annu Rev Biomed Eng. 2005;7:55–76.
Chan WC, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281(5385):2016–8.
Li ZG, Yang K, Cao YA, Zheng G, Sun DP, Zhao C, et al. In vivo study of the effects of peptide-conjugated near-infrared fluorescent quantum dots on the tumorigenic and lymphatic metastatic capacities of squamous cell carcinoma cell line Tca8113 and U14. Int J Mol Sci. 2010;11(4):1413–22.
Nurunnabi M, Cho KJ, Choi JS, Huh KM, Lee YK. Targeted near-IR QDs-loaded micelles for cancer therapy and imaging. Biomaterials. 2010;31(20):5436–44.
Huisman HJ, Fütterer JJ, van Lin ENJT, Welmers A, Scheenen TWJ, van Dalen JA, et al. Prostate cancer: Precision of integrating functional MR imaging with radiation therapy treatment by using fiducial gold markers. Radiology. 2005;236(1):311–7.
Ackerson CJ, Sykes MT, Kornberg RD. Defined DNA/nanoparticle conjugates. Proc Natl Acad Sci U S A. 2005;102(38):13383–5.
Huang X, El-Sayed IH, Qian W, El-Sayed MA. cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128(6):2115–20.
Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, et al. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Can Res. 2003;63(9):1999–2004.
Yelin D, Oron D, Thiberge S, Moses E, Silberberg Y. Multiphoton plasmon-resonance microscopy. Opt Express. 2003;11(12):1385–91.
Troutman TS, Barton JK, Romanowski M. Optical coherence tomography with plasmon resonant nanorods of gold. Opt Lett. 2007;32(11):1438–40.
Trowbridge IS, Omary MB. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981;78(5):3039–43.
Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15(4):392–400.
Ji S, Xu J, Zhang B, Yao W, Xu W, Wu W, et al. RGD-conjugated albumin nanoparticles as a novel delivery vehicle in pancreatic cancer therapy. Cancer Biol Ther. 2012;13(4):206–15.
Cai W, Chen X. Preparation of peptide-conjugated quantum dots for tumor vasculature-targeted imaging. Nat Protoc. 2008;3(1):89–96.
Tikhomirov G, Hoogland S, Lee PE, Fischer A, Sargent EH, Kelley SO. DNA-based programming of quantum dot valency, self-assembly and luminescence. Nat Nanotechnol. 2011;6(8):485–90.
Ma N, Sargent EH, Kelley SO. One-step DNA-programmed growth of luminescent and biofunctionalized nanocrystals. Nat Nanotechnol. 2009;4(2):121–5.
He X, Gao L, Ma N. One-step instant synthesis of protein-conjugated quantum dots at room temperature. Sci Rep. 2013;3:2825.
Gillis P, Koenig SH. Transverse relaxation of solvent protons induced by magnetized spheres: Application to ferritin, erythrocytes, and magnetite. Magn Reson Med. 1987;5(4):323–45.
Felsher DW. Cancer revoked: Oncogenes as therapeutic targets. Nat Rev Cancer. 2003;3(5):375–80.
Triesscheijn M, Baas P, Schellens JH, Stewart FA. Photodynamic therapy in oncology. Oncologist. 2006;11(9):1034–44.
Pagonis TC, Chen J, Fontana CR, Devalapally H, Ruggiero K, Song X, et al. Nanoparticle-based endodontic antimicrobial photodynamic therapy. J Endod. 2010;36(2):322–8.
Park JH, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Micellar hybrid nanoparticles for simultaneous magnetofluorescent imaging and drug delivery. Angew Chem Int Ed Engl. 2008;47(38):7284–8.
Singh SP. Multifunctional magnetic quantum dots for cancer theranostics. J Biomed Nanotechnol. 2011;7(1):95–7.
Ye F, Barrefelt A, Asem H, Abedi-Valugerdi M, El-Serafi I, Saghafian M, et al. Biodegradable polymeric vesicles containing magnetic nanoparticles, quantum dots and anticancer drugs for drug delivery and imaging. Biomaterials. 2014;35(12):3885–94.
Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol Med. 1995;18(2):321–36.
Prakash AS, Rao KS, Dameron CT. Cadmium inhibits BPDE alkylation of DNA in the major groove but not in the minor groove. Biochem Biophys Res Commun. 1998;244(1):198–203.
Hossain Z, Huq F. Studies on the interaction between Cd2+ ions and DNA. J Inorg Biochem. 2002;90:85–96.
Beyersmann D, Hechtenberg S. Cadmium, gene regulation, and cellular signalling in mammalian cells. Toxicol Appl Pharmacol. 1997;144(2):247–61.
Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4(1):11–8.
Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattoussi H, Ober R, et al. (CdSe)ZnS core−shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J Phys Chem B. 1997;101(46):9463–75.
Qu L, Peng ZA, Peng X. Alternative routes toward high quality CdSe nanocrystals. Nano Lett. 2001;1(6):333–7.
Yong KT, Hu R, Roy I, Ding H, Vathy LA, Bergey EJ, et al. Tumor targeting and imaging in live animals with functionalized semiconductor quantum rods. ACS Appl Mater Interfaces. 2009;1(3):710–9.
Winter JO, Liu TY, Korgel BA, Schmidt CE. Recognition molecule directed interfacing between semiconductor quantum dots and nerve cells. Adv Mater. 2001;13(22):1673–7.
Guisinger NP, Arnold MS. Beyond silicon: Carbon-based nanotechnology. MRS Bull. 2010;35(4):273–9.
Demchenko AP, Dekaliuk MO. Novel fluorescent carbonic nanomaterials for sensing and imaging. Methods Appl Fluoresc. 2013;1(4): 042001.
Baker SN, Baker GA. Luminescent carbon nanodots: Emergent nanolights. Angew Chem Int Ed Engl. 2010;49(38):6726–44.
Sun Y-P, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc. 2006;128(24):7756–7.
Zheng L, Chi Y, Dong Y, Lin J, Wang B. Electrochemiluminescence of water-soluble carbon nanocrystals released electrochemically from graphite. J Am Chem Soc. 2009;131(13):4564–5.
Li X, Wang H, Shimizu Y, Pyatenko A, Kawaguchi K, Koshizaki N. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem Commun (Camb). 2011;47(3):932–4.
Liu H, Ye T, Mao C. Fluorescent carbon nanoparticles derived from candle soot. Angew Chem. 2007;119(34):6593–5.
Wang F, Pang S, Wang L, Li Q, Kreiter M, Liu C. One-step synthesis of highly luminescent carbon dots in noncoordinating solvents. Chem Mater. 2010;22(16):4528–30.
Wang X, Qu K, Xu B, Ren J, Qu X. Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J Mater Chem. 2011;21(8).
El Essawy NA, Konsowa AH, Elnouby M, Farag HA. A novel one-step synthesis for carbon-based nanomaterials from polyethylene terephthalate (PET) bottles waste. J Air Waste Manag Assoc. 2017;67(3):358–70.
Ding C, Zhu A, Tian Y. Functional surface engineering of C-dots for fluorescent biosensing and in vivo bioimaging. Acc Chem Res. 2014;47(1):20–30.
Zhu S, Song Y, Zhao X, Shao J, Zhang J, Yang B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res. 2015;8(2):355–81.
Ma DL, Lin S, Wang W, Yang C, Leung CH. Luminescent chemosensors by using cyclometalated iridium(iii) complexes and their applications. Chem Sci. 2017;8(2):878–89.
Liu J, Zhang P, Yang X, Wang K, Guo Q, Huang J, et al. Aptamer-mediated indirect quantum dot labeling and fluorescent imaging of target proteins in living cells. Nanotechnology. 2014;25(50): 505502.
DuChene JS, Sweeny BC, Johnston-Peck AC, Su D, Stach EA, Wei WD. Prolonged hot electron dynamics in plasmonic-metal/semiconductor heterostructures with implications for solar photocatalysis. Angew Chem Int Ed Engl. 2014;53(30):7887–91.
Wang N, Yu X, Zhang K, Mirkin CA, Li J. Upconversion nanoprobes for the ratiometric luminescent sensing of nitric oxide. J Am Chem Soc. 2017;139(36):12354–7.
Jiang Y, Wang M, Hardie J, Tonga GY, Ray M, Xu Q, et al. Chemically engineered nanoparticle-protein interface for real-time cellular oxidative stress monitoring. Small. 2016;12(28):3775–9.
Li H, Shao F-Q, Zou S-Y, Yang Q-J, Huang H, Feng J-J, et al. Microwave-assisted synthesis of N, P-doped carbon dots for fluorescent cell imaging. Microchim Acta. 2015;183(2):821–6.
Cai QY, Li J, Ge J, Zhang L, Hu YL, Li ZH, et al. A rapid fluorescence “switch-on” assay for glutathione detection by using carbon dots-MnO2 nanocomposites. Biosens Bioelectron. 2015;72:31–6.
Dong Y, Pang H, Yang HB, Guo C, Shao J, Chi Y, et al. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew Chem Int Ed Engl. 2013;52(30):7800–4.
Tang F, Wang C, Wang X, Li L. Facile synthesis of biocompatible fluorescent nanoparticles for cellular imaging and targeted detection of cancer cells. ACS Appl Mater Interfaces. 2015;7(45):25077–83.
Qiu J, Zhang R, Li J, Sang Y, Tang W, Rivera Gil P, et al. Fluorescent graphene quantum dots as traceable, pH-sensitive drug delivery systems. Int J Nanomedicine. 2015;10:6709–24.
Leamon CP, Low PS. Delivery of macromolecules into living cells: A method that exploits folate receptor endocytosis. Proc Natl Acad Sci U S A. 1991;88(13):5572–6.
Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338(2):284–93.
Song Y, Shi W, Chen W, Li X, Ma H. Fluorescent carbon nanodots conjugated with folic acid for distinguishing folate-receptor-positive cancer cells from normal cells. J Mater Chem. 2012;22(25).
Elliott RL, Head JF. Cancer: Tumor iron metabolism, mitochondrial dysfunction and tumor immunosuppression; “A tight partnership—Was warburg correct?” J Cancer Ther. 2012;03(04):278–311.
Tortorella S, Karagiannis TC. Transferrin receptor-mediated endocytosis: A useful target for cancer therapy. J Membr Biol. 2014;247(4):291–307.
Fritzer M, Barabas K, Szüts V, Berczi A, Szekeres T, Faulk WP, et al. Cytotoxicity of a transferrin-adriamycin conjugate to anthracycline-resistant cells. Int J Cancer. 1992;52(4):619–23.
Lubgan D, Jozwiak Z, Grabenbauer GG, Distel LV. Doxorubicin-transferrin conjugate selectively overcomes multidrug resistance in leukaemia cells. Cell Mol Biol Lett. 2009;14(1):113–27.
Fritzer M, Szekeres T, Szüts V, Jarayam HN, Goldenberg H. Cytotoxic effects of a doxorubicin-transferrin conjugate in multidrug-resistant KB cells. Biochem Pharmacol. 1996;51(4):489–93.
Acknowledgements
The authors acknowledge the infrastructural facilities provided by NIT Rourkela.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mukherjee, A., Sarkar, N. (2022). Recent Developments in Quantum Dots Technologies as Effective Theranostic Tools Against Cancer. In: Barik, P., Mondal, S. (eds) Application of Quantum Dots in Biology and Medicine. Springer, Singapore. https://doi.org/10.1007/978-981-19-3144-4_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-3144-4_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3143-7
Online ISBN: 978-981-19-3144-4
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)