Abstract
Cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage are among the major clinical concerns for which effective therapies are poorly available. Accumulating lines of evidence indicate that drugs acting on nicotinic acetylcholine receptors (nAChRs) may provide therapeutic effects on these disorders, based on their neuroprotective and anti-inflammatory actions. For example, the cholinergic neurotransmission in the central nervous system via nAChRs may function as an endogenous neuroprotective system that prevents pathogenic events associated with ischemic stroke. On the other hand, exogenous administration of nicotine or nAChR agonists to experimental models of ischemic stroke has been reported to produce conflicting results (either protective or deleterious), which may be largely dependent on the different regiments of drug treatments. With regard to intracerebral hemorrhage, preclinical findings suggest that post-treatment with nAChR agonists is effective in alleviating brain tissue damage and neurological outcome. The beneficial actions of nAChR agonist have also been reported for an experimental model of subarachnoid hemorrhage, which should be confirmed by further investigations. Although smoking has been considered as an important risk factor for stroke episodes, specific targeting of the central nAChRs may prove to be an effective and novel strategy for the treatment of diverse types of cerebrovascular disorders.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Nicotine
- Cerebral infarction
- Hemorrhagic stroke
- Neuroprotection
- Neuroinflammation
- Acethylcholinesterase inhibitor
7.1 Introduction
Acetylcholine (ACh) has a long history of biomedical research as it is the first compound that has been identified as a chemical neurotransmitter. In the periphery, motor neurons release ACh from their nerve terminals that innervate skeletal muscles and transmit signals via activation of muscle type nicotinic acetylcholine receptors (nAChRs) that are heteropentamers consisting of subunits named α1, β1, δ and ε. The cell bodies of sympathetic and parasympathetic postganglionic neurons express neuronal type of nAChRs composed of subunits distinct from those contained in muscle nAChRs, such as α3α5β4 and α3α5β2β4. These nAChRs expressed in autonomic ganglia receive cholinergic inputs from preganglionic neurons. Postganglionic neurons of the parasympathetic nervous system also utilize ACh as a neurotransmitter, and various peripheral tissues receive the signals from these neurons mainly via muscarinic ACh receptors.
Cholinergic neurotransmitter system is also present in the brain, and nAChRs as well as muscarinic receptors are widely distributed throughout the central nervous system (Taly et al. 2009). A notable feature of nAChR-mediated signal transmission in the central nervous system is that it may mediate various functions in addition to the role in fast neurotransmission. That is, stimulation of nAChRs in central neurons affords cytoprotective and survival-promoting effects, as discussed in other chapters as well as in the literatures elsewhere (Akaike et al. 2010; Mudo et al. 2007). In addition, nAChR stimulation may be able to limit inflammation in the brain under pathological conditions, by directly regulating the cells involved in inflammatory reactions such as brain-resident microglia and infiltrating monocytes/macrophages (Shytle et al. 2004; Wang et al. 2003). The principal nAChR subtypes expressed in the central nervous system are homopentamers consisting of α7 subunits and heteropentamers consisting of α4 and β2 subunits, although nAChRs containing other types of subunits such as α3, α6, β3 and β4 are also expressed in a brain region-specific manner (Taly et al. 2009; Zoli et al. 2015). Differences in subunit compositions between the peripheral nAChRs (either muscle type or neuronal type) and the central nAChRs may enable drug development specifically targeted for nAChRs in the brain, thereby avoiding adverse peripheral actions.
One of the major reasons for the central nAChRs being expected to serve as drug targets is that effective pharmacotherapies are not available at present for various kinds of central nervous system disorders associated with neurodegeneration. Among these disorders are cerebrovascular diseases resulting from the disruption of circulatory system that supplies blood flow to the brain. This chapter summarizes various preclinical and clinical findings related to the functions of nAChRs in regulating pathological events associated with cerebrovascular disorders, and discusses the potentials of nAChR-related drugs as novel therapeutics combating these disorders.
7.2 Overviews on Stroke Disorders
The term “stroke” represents diverse sets of disorders associated with disrupted cerebrovascular functions, which are classified into two major categories such as ischemic stroke and hemorrhagic stroke. Ischemic stroke is featured by interception of the blood flow in the brain tissues and, depending on the causes of interception, is further categorized into several different types including atherothrombotic brain infarction, cardiogenic embolism and lacunar stroke. In either case, the consequences of hypoperfusion are shortage of supplies of oxygen and glucose to the brain parenchymal cells. Because energy demands of neurons are met almost entirely by the aerobic metabolism of glucose, even short periods of ischemic episodes may result in severe and irreversible brain tissue damage accompanied by neuron loss. Rescuing neurons from cell death is extremely difficult in the ischemic core region where oxygen/glucose supply is almost totally abolished. In contrast, in the peri-infarct or “penumbra” region where oxygen/glucose supply is decreased but not abolished, neuronal cell death displays at least in part the features of programmed cell death that occurs with a certain delay from the onset of the insults. Therefore, neuroprotection in the penumbra after ischemic insults may be possible if appropriate treatment strategies are established (Catanese et al. 2017). Practically, however, only a few drugs effective in brain tissue preservation are available at present for the treatment of ischemic stroke (Table 7.1). A powerful approach is the reestablishment of blood flow by removal of the blood clot, and for this purpose, tissue plasminogen activator that promotes fibrinolysis is administered within 4.5 h after ischemic attack. Otherwise, edaravone is the sole choice of drugs in Japan intended for neuroprotection via its free radical-scavenging action.
The major types of hemorrhagic stroke are characterized by extravasation of blood into either brain parenchyma (intracerebral hemorrhage; ICH) or subarachnoid space (subarachnoid hemorrhage; SAH), and both cases are accompanied by poor prognosis. Hemorrhagic stroke has pathological features quite distinct from those of ischemic stroke. In ICH, tissue damage is caused primarily by the blood flowing out of ruptured vessels into the brain parenchyma. Biological actions of blood constituents (particularly, proteases involved in the coagulation cascade), in addition to the physical damage of blood mass (so called the “mass effect”) are the main contributors of pathogenic events in ICH (Qureshi et al. 2009). Currently, there are no established pharmacotherapies that are applicable after the occurrence of ICH events and are effective in neuroprotection (Katsuki 2010). Drugs such as glycerol and mannitol that manipulate plasma osmotic pressure and suppress brain edema formation may be available, but they still lack solid clinical evidence that justifies their application to ICH cases (Table 7.1). Clinical practice for SAH is in a similar situation to that for ICH, in the sense that direct neuroprotective pharmacotherapies are yet unavailable. Representative drugs used for the current treatment of SAH include fasudil, a Rho kinase inhibitor that prevents post-SAH vasospasm and ozagrel, an inhibitor of thromboxane A2 synthase that prevents platelet aggregation.
Overall, although cerebrovascular disorders such as ischemic stroke, ICH and SAH are among the major issues of clinical concerns in the modern world, effective drug treatment strategies are, for the most part, far from established. With these situations under consideration, the following sections summarize the findings obtained from various kinds of efforts addressing the possibility of nAChRs as drug targets for the therapies of these disorders.
7.3 Ischemic Stroke and nAChRs
7.3.1 Roles of Endogenous Cholinergic System in Regulation of Ischemic Injury
7.3.1.1 Effects of nAChR Antagonists and Allosteric Modulators
Potential involvement of endogenous ACh in regulation of the pathogenic events in ischemic brain injury has been suggested by a study addressing the effects of nAChR antagonists in neonatal rats. In 7-day-old rats that underwent permanent occlusion of left common carotid artery, subcutaneous administration of subtype non-selective nAChR antagonist mecamylamine or specific α7 nAChR antagonist methyllycaconitine just before induction of 1-h hypoxia aggravated neural damage in the hippocampus. Mecamylamine also aggravated neural damage in the cerebral cortex (Furukawa et al. 2013). On the other hand, daily treatment with mecamylamine from 24 h after induction of 45-min transient global ischemia did not clearly affect the degree of cell death-related events such as the number of dead CA1 neurons and caspase-3 activity in the hippocampus in young adult mice (Ray et al. 2014).
PNU-120596 is a positive allosteric modulator of α7 nAChRs. The drug does not activate nAChRs by itself but inhibits desensitization and enhances activation of α7 nAChRs by orthosteric agonists. In addition, the ACh degradation product choline may produce agonistic activity at nAChRs in the presence of positive allosteric modulators. Indeed, Kalappa et al. (2013) demonstrated that choline delayed oxygen/glucose deprivation-induced injury of hippocampal CA1 neurons in vitro in the presence of PNU-120596, and PNU-120596 alone was not effective in the absence of choline in these experimental settings. The combined neuroprotective effect of choline and PNU-120596 was abolished by α7 nAChR antagonist methyllycaconitine. The neuroprotective potential of PNU-120596 was also confirmed in 90-min transient focal ischemia in rats, where 3-h pretreatment or 30-min posttreatment with the drug significantly reduced the infarct volume (Kalappa et al. 2013). A companion study by the same group reported that the effective time window of PNU-120596 administered intravenously was extended to 6 h after induction of middle cerebral artery occlusion (MCAO; Sun et al. 2013). These results strongly suggest that cholinergic neurotransmission functions as an endogenous neuroprotective system against ischemic brain injury.
7.3.1.2 Effects of Acetylcholinesterase Inhibitors
The concentration of ACh in the synaptic cleft is tightly regulated by acetylcholinesterase (AChE), as ACh released from the nerve terminals is promptly degraded by this hydrolyzing enzyme. Accordingly, application of AChE inhibitors is a conventional procedure to augment cholinergic neurotransmission. Several studies have addressed the effect of AChE inhibitors in rodent models of ischemic stroke. The earliest example is a study on donepezil, a centrally acting reversible inhibitor of AChE that is prescribed for amelioration of cognitive deficits in Alzheimer disease. An oral dose of donepezil (12 mg/kg) administered 2 h before or 1 h after induction of permanent MCAO in rats significantly reduced the infarct volume. The protective effect of donepezil was abolished by mecamylamine, suggesting the involvement of nAChR activation (Fujiki et al. 2005).
Another AChE inhibitor galantamine has also been reported to exhibit neuroprotective effects where the first administration was performed 24 h before or 3 h after induction of transient global ischemia in Mongolian gerbils by occlusion of the common carotid artery. The effects included an increase in surviving neurons, a decrease in terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive cells/caspase-3-positive cells in hippocampal CA1 region and amelioration of spatial memory deficit, and these effects were attenuated by concurrent treatment with mecamylamine (Lorrio et al. 2007). A later examination using rat hippocampal slices subject to oxygen/glucose deprivation followed by reoxygenation revealed that a tyrosine kinase Janus kinase (Jak) 2 mediated the protective effect of galantamine by suppressing activation of nuclear factor (NF) -κB and induction of inducible nitric oxide synthase (iNOS) expression (Egea et al. 2012). Under similar experimental conditions in mouse hippocampal slices, 30-min pretreatment with nicotine (10–100 μM) reduced the extent of oxygen/glucose deprivation-induced cell injury via activation of α7 nAChRs (Egea et al. 2007).
Wang et al. (2008) reported the effect of huperzine A, yet another AChE inhibitor, in a rat model of transient focal cerebral ischemia. When administered at the onset or 6 h after transient MCAO, huperzine A restored regional cerebral blood flow, reduced infarct size and decreased neurological deficit score. Similar to the cases with galantamine, huperzine A inhibited NF-κB activation and decreased expression of proinflammatory factors such as tumor necrosis factor (TNF) -α, interleukin (IL) -1β, iNOS and cyclooxygenase (COX) -2. In addition, mecamylamine abolished the inhibitory effect of huperzine A on glial cell activation and partially reversed the reduction of infarct size by huperzine A.
Overall, these results obtained from the examinations on three different kinds of AChE inhibitors are consistent with the idea that endogenous ACh affords neuroprotective effects against ischemia and ischemia-reperfusion injury, by activating nAChRs (Fig. 7.1).
Enhancement of endogenous cholinergic signaling suppresses ischemia-induced pathological events. Stimulation of nicotinic acetylcholine receptor (nAChR) by endogenous acetylcholine (ACh) can be enhanced either by inhibitors of acetylcholinesterase (AChE) such as donepezil that promote accumulation of extracellular ACh, or by positive allosteric modulators (PAMs) of nAChRs such as PNU-120596 that increase the affinity and/or efficacy of ACh on nAChRs. PAMs may also allow choline to exert agonistic activity on nAChRs. Stimulation of nAChRs, particularly of α7 subtype, by these drugs leads to phosphorylation/activation of a tyrosine kinase Janus kinase 2 (Jak2). Although detailed mechanisms are yet to be identified, activated Jak2 may inhibit phosphorylation of inhibitor of κB (IκB) α, prevent ischemia-induced recruitment of nuclear factor κB (NF-κB; illustrated as subunits p65 and p50), and consequently, suppress NF-κB-dependent expression of pro-inflammatory factors (See Sects. 7.3.1.1 and 7.3.1.2 for details)
7.3.1.3 Effect of Cholinergic Neuronal Activity
Vagus nerve stimulation is known to produce several therapeutic effects, and in the case of ischemia/reperfusion injury in rats, a brief episode of vagus nerve stimulation ameliorates neurological deficits and reduces infarct volume (Sun et al. 2012). Jiang et al. (2014) addressed the mechanisms of the neuroprotective effects of vagus nerve stimulation. They confirmed that vagus nerve stimulation given at 30 min after transient focal ischemia was able to ameliorate neurological deficit and reduced the infarct volume at 24 h after reperfusion. They additionally found that the increase in the levels of several cytokines such as TNF-α, IL-1β and IL-6 in the peri-infarct region was significantly prevented by vagus nerve stimulation. Notably, vagus nerve stimulation prevented ischemia/reperfusion-induced loss of α7 nAChR expression in microglia in the penumbra region, and also reversed ischemia-induced decrease in the level of phosphorylated Akt. Although the evidence is at most indirect, these results imply the involvement of cholinergic neurotransmission pathway in the neuroprotective effect of vagus nerve stimulation.
As for microglial nAChRs, another line of findings in a positron emission tomography imaging study implicates α4β2 nAChRs in the pathology of cerebral ischemia. That is, α4β2 nAChR binding probed by 2[18F]-fluoro-A85380 and [11C]PK11195 in rat brains showed a transient increase that peaked at 7 days after MCAO (Martin et al. 2015). Increased expression of α4β2 nAChRs in microglia/macrophages and astrocytes was confirmed by immunohistochemical examinations. Although the functional significance of these changes in the expression of α4β2 nAChRs is unclear, this nAChR subtype in addition to α7 nAChRs may serve as a target for regulation of pathogenic events, particularly those involving inflammatory reactions, in ischemic brain injury.
7.3.2 Effects of nAChR Agonists on Ischemic Injury
7.3.2.1 Positive Findings
As discussed in the previous section (Sect. 7.3.1), stimulation of nAChRs appears to provide neuroprotective effects against ischemia-reperfusion injury. Indeed, many lines of evidence indicate that nicotine and other compounds with direct agonistic activity on nAChRs can provide beneficial effects on experimental model of ischemic stroke (Table 7.2). Kagitani et al. (2000) reported the effect of intravenous injection of nicotine in an intermittent transient ischemia model in rats. When rats with permanent ligation of bilateral vertebral arteries received transient and intermittent occlusion (two to three times for 2 min at 2-min intervals) of bilateral carotid arteries, the blood flow in the hippocampus was substantially and reversibly decreased, and delayed death of hippocampal CA1 neurons was induced. Administration of nicotine (30–100 μg/kg) at 5 min before occlusion slightly but significantly improved hippocampal blood flow during occlusion and increased the number of surviving CA1 neurons. Kagitani et al. (2000) assume that the neuroprotecive effect of nicotine under these conditions is attributable to the vasodilative response induced by nAChR stimulation and resultant increase in regional blood flow in the hippocampus. On the other hand, the presence of nicotine during ischemic episodes may produce direct protective effects on neuronal cells. For example, in rat primary cortical cultures, 4-h hypoxia induced neuronal cell death with features of apoptosis. Application of nicotine (10–100 μM) during hypoxia prevented cell death probably via activation of both α7 nAChRs and α4β2 nAChRs, as methyllycaconitine (α7 nAChR antagonist) and dihydro-β-erythroidine (α4β2 nAChR antagonist) blocked the effect of nicotine (Hejmadi et al. 2003).
GTS-21 is a synthetic nAChR agonist with fourfold higher affinity than nicotine for α7 nAChRs. The effect of GTS-21 and nicotine was tested on an ischemia-reperfusion model of Mongolian gerbils. When the drugs were injected intraperitoneally at 30 min before 3-min forebrain ischemia, both GTS-21 (1–5 mg/kg) and nicotine (0.3–1.5 mg/kg) improved performance of animals in passive avoidance and attenuated cell death in the hippocampal CA1 region. GTS-21 (10 mg/kg) was effective also when orally administered twice daily for 2 weeks prior to ischemia (Nanri et al. 1998b). Moreover, the same group examined the effect of GTS-21 in rats with permanent occlusion of bilateral common carotid arteries (Nanri et al. 1998a). In this study, GTS-21 (1 or 10 mg/kg) was administered orally 24 h and 30 min before induction of ischemia and then once daily for 2 months. GTS-21 significantly prevented ischemia-induced histopathological changes in the cerebral cortex and in the white matter, and improved the cognitive function of rats as assessed by radial maze learning performance.
Potential involvement of endocannabinoid system in the neuroprotective effect of nicotine has been proposed by a study by Chen et al. (2013). Intraperitoneal administration of nicotine hydrogen tartrate (1.2 mg/kg) transiently increased the tissue content of endocannabinoids 2-arachidonoylglycerol and anandamide in rats, which peaked at 2 h after administration. Nicotine also increased the protein expression level of cannabinoid CB1 receptor. Moreover, 2-h pretreatment with nicotine reduced the infarct volume and neurological deficit induced by 120-min MCAO, and these effects were significantly reversed by AM251, a CB1 receptor antagonist.
All of these findings mentioned above were obtained by pretreatment of nicotine or nAChR agonist. However, the pretreatment regimens of drugs are not very useful for combating stroke disorders, because in almost all clinical situations the drug therapy is available only after certain delay from the onset of acute stroke episodes. With these concerns, several studies have examined the effect of therapeutic treatment with nAChR agonists on ischemic injury and indeed reported positive results. In a study by Guan et al. (2015), transient forebrain ischemia was induced in rats by four-vessel occlusion. Nicotine (0.5 mg/kg) was administered intraperitoneally at 2, 6 or 12 h after induction of 15-min ischemia, and thereafter, three times per day for 7 days. Under these conditions, nicotine significantly increased the number of surviving CA1 neurons in the hippocampus. At the same time, nicotine was found to decrease the number of microglia and to prevent ischemia-induced up-regulation of mRNAs encoding TNF-α and IL-1β in the hippocampal CA1 region. In addition, nicotine inhibited proliferation of primary cultured microglia, and this effect was blocked by α7 nAChR antagonist α-bungarotoxin. These results suggest that attenuation of proliferation and activation of microglia contribute to the neuroprotective effect of the therapeutic treatment with nicotine.
Involvement of regulation of microglia in the therapeutic effect of α7 nAChR agonist has also been demonstrated in a photothrombotic ischemia model in mice (Parada et al. 2013). That is, intraperitoneal administration of PNU-282987 (10 mg/kg) at 60 min after induction of thrombotic stroke using Rose Bengal decreased the volume of cortical infarct and diminished neurological deficits. Notably, the protective effect of PNU-282987 was abolished by concomitant treatment with heme oxygenase (HO) inhibitor or by deletion of HO-1 gene. In hippocampal slice culture preparation, PNU-282987 induced HO-1 expression, prevented cell death and attenuated production of reactive oxygen species and TNF-α resulting from oxygen/glucose deprivation and reoxygenation. The protective effect of PNU-282987 in slice cultures was abolished by application of HO inhibitor or by depletion of microglia from the slices. These results propose HO-1 as a key mediator of the neuroprotective and anti-inflammatory effect of microglial α7 nAChR stimulation (Fig. 7.2).
Specific α7 nAChR agonist may afford anti-inflammatory effects via expression of heme oxygenase-1 (HO-1) in the brains after ischemic insults. According to Parada et al. (2010, 2013), application of α7 nAChR agonist such as PNU-282987 activates Jak2 in microglia/macrophages, which leads to activation of PI3-kinase (PI3K)/Akt pathway. By the action of Akt, a transcription factor Nrf2 is liberated from Keap1, escapes from degradation and binds to antioxidant response element (ARE) of the promoter regions of genes encoding phase II detoxifying enzymes such as HO-1 and the catalytic subunit of glutamate cysteine ligase (GCL-c). HO-1 induced by these signaling pathways plays a critical role in inhibiting ischemia-associated pro-inflammatory responses such as the production of reactive oxygen species (ROS) and tumor necrosis factor-α (TNF-α)
Using another α7 nAChR agonist PHA-568487, Han et al. (2014b) examined the role of microglia/macrophage phenotypes. Mice were treated with PHA-568487 immediately and 24 h after permanent MCAO. The treatment significantly reduced the infarct volume and partially ameliorated behavioral performance. The peri-infarct region of PHA-568487-treated mice contained a smaller number of pro-inflammatory microglia/macrophages (so called “M1” phenotype identified as CD11b+Iba-1+) and a larger number of anti-inflammatory microglia/macrophages (so called “M2” phenotype identified as CD206+Iba-1+), than that of saline-treated mice (Han et al. 2014b). Similar protective effect on neuron survival and regulatory effect on microglia/macrophage phenotypes were obtained when administration of PHA-568487 (0.8 mg/kg) was initiated at 1 day after permanent MCAO (Han et al. 2014a). These results suggest that the regulation of microglia/macrophage phenotypes underlies the neuroprotective effect of α7 nAChR agonist against ischemic injury.
The effect of repeated administration (twice daily for 12 days) of low dose nicotine (0.3 mg/kg as nicotine hydrogen tartrate) has been reported in a rat focal ischemia model prepared by unilateral devascularization in the motor cortex. The findings are notable in the sense that post-treatment started as late as 48 h after ischemic surgery was proven to be effective in promoting the recovery of motor performance. Interestingly, nicotine treatment increased the morphological complexity of dendritic processes of layer V pyramidal neurons of the cingulate cortex, both in control rats and ischemia-induced rats (Gonzalez et al. 2006). Therefore, nicotine-induced enhancement of dendritic growth of cortical neurons might facilitate the recovery of motor functions.
7.3.2.2 Negative Findings
Under several experimental conditions, nicotine has been reported to produce no effect or even worsen the outcome of ischemic events. For example, Lim et al. (2009) examined the effect of nicotine on rehabilitation following devascularization in the forelimb area of the motor cortex in rats. Oral administration of nicotine (0.3 mg/kg, twice daily for 3 weeks starting from 1 week before stroke induction) did not improve motor function recovery.
It should be noted that virtually all studies reporting the deleterious effect of nicotine on ischemic brain injury were aimed to reveal potential adverse effects of smoking on cerebrovascular disorders, and therefore that these studies generally employ long-term and/or continuous pretreatment regimen for nicotine administration (Table 7.3). An early study by Wang et al. (1997) examined the effect of nicotine (4.5 mg/kg/day) administered subcutaneously for 14 days by osmotic minipumps, prior to induction of ischemic insults in rats. Nicotine decreased the blood flow in the penumbra region during reperfusion after transient MCAO, worsened neurological deficit, and increased the injury and edema volume. Interestingly, nicotine-treated rats exhibited depletion of tissue plasminogen activator in cerebral capillaries, which might be causally relevant to the poor recovery of the blood flow after reperfusion and to the resultant exacerbation of brain tissue injury. In a more recent study, the effect of 4-week continuous treatment with nicotine (2 or 4 mg/kg/day, subcutaneously by osmotic pumps) prior to 2-h transient MCAO was examined (Li et al. 2016). Nicotine at both doses significantly increased the infarct size, although the neurological deficit seems to be ameliorated by the treatments. Authors attributed the exacerbation of pathological changes by nicotine to enhanced oxidative stress, as the cerebral cortex and the cerebral arteries in nicotine-treated rats exhibited decreased expression levels of Mn superoxide dismutase and mitochondrial uncoupling protein-2, as compared to those in control rats.
Bradford et al. (2011) examined the effect of chronic nicotine treatment with special reference to inflammatory responses. In their study, 14-day pretreatment with nicotine (0.5–2 mg/kg/day, subcutaneously by osmotic pumps) dose-dependently increased the infarct volume and brain water content at 3 days after 30-min MCAO. In addition, nicotine given at 2 mg/kg/day worsened neurological outcome and decreased the survival rate of mice. They also demonstrated that 14-day treatment with nicotine (2 mg/kg/day), either alone or in combination with ischemia/reperfusion insults, markedly increased the expression of various cytokines (including TNF-α and IL-1β) and chemokines (including CCL2 and CXCL5) in brain tissue and isolated brain microvessels. Consistent with these observations, ischemia/reperfusion-induced infiltration of neutrophils and monocytes was augmented by 14-day pretreatment with nicotine.
In a study on permanent MCAO in mice, subcutaneous delivery of 4.5 mg/kg/day for 3 weeks has been shown to exacerbate brain edema (Paulson et al. 2010). Exacerbation of brain edema was also observed when nicotine was intraperitoneally administered 1 h before MCAO. Although the precise mechanisms of action of nicotine in these experimental settings have not been addressed, the effect might be relevant to nicotine-induced altered functions of Na+, K+, 2Cl− cotransporters in the blood-brain barrier (Paulson et al. 2006).
Potential interaction of the effect of nicotine with that of estrogen has been proposed by studies using ovariectomized rats. Daily intraperitoneal injection of 1 mg/kg nicotine for 15 days to female rats with normal menstrual cycle accelerated neuron loss in the hippocampal CA1 region at 7 days after 10-min ligation of carotid artery. In addition, ovariectomy per se exacerbated ischemia-induced loss of CA1 neurons and occluded the effect of repeated nicotine treatment (Ravel et al. 2009). These results suggest that the neuroprotective effect of endogenous estrogen is cancelled by nicotine. Indeed, oral contraceptives that lowered the plasma level of 17β-estradiol exacerbated ischemia-induced loss of hippocampal CA1 neurons, synergistically with 16-day treatment of nicotine. Abolishment by nicotine of the neuroprotective effect of 17β-estradiol on oxygen/glucose deprivation-induced injury, as well as attenuation by nicotine of estrogen receptor-mediated recruitment of cyclic AMP response element binding protein, was also demonstrated in rat hippocampal slice cultures (Ravel et al. 2011).
Finally, neonatal male rats continuously exposed to nicotine during the perinatal period (from day 4 of gestation to day 10 after birth) exhibited larger infarct size after hypoxic-ischemic injury than control rats. The augmented pathology may be attributable to altered angiotensin II signaling resulting from decreased expression of angiotensin II AT2 receptors (Li et al. 2012).
Taken together, long-term continuous exposure to nicotine may aggravate the consequences of ischemic stroke, by altering expression and function of various cytoprotective proteins, pro-inflammatory factors, transporters and receptors in the brain (Fig. 7.3).
Nicotine may produce harmful influences on ischemia-related pathological events. Long-term pretreatment with nicotine by continuous infusion is generally shown to aggravate the pathology of ischemic stroke in experimental animals. Multiple mechanisms are postulated to play important roles in these harmful consequences as summarized here (See Sect. 7.3.2.2 for details. SOD superoxide dismutase, t-PA tissue plasminogen activator, UCP-2 uncoupling protein-2)
7.4 Hemorrhagic Stroke and nAChRs
7.4.1 Effects of nAChR Agonists on Intracerebral Hemorrhage (ICH)
ICH is initiated by the rupture of blood vessels within the brain parenchyma and bleeding from ruptured vessels. The mechanisms involved in brain tissue injury after ICH include several distinct features from those involved in ischemic brain injury. A notable feature of ICH is that blood constituents may exert specific biological actions on neurons and glial cells in the brain and make substantial contribution to the complicated pathogenic events. Thrombin, a serine protease that normally functions in the coagulation cascade, is a remarkable example because it can stimulate several members of protease-activated receptors (PARs) that are expressed in various cell types including neurons, astrocytes and microglia. Indeed, several lines of evidence indicate that thrombin may contribute to the pathogenesis of experimental ICH models. For example, a thrombin inhibitor argatroban reduces edema and inflammation associated with ICH in rats (Kitaoka et al. 2002; Nagatsuna et al. 2005).
To facilitate investigations on the pathogenic mechanisms of ICH and exploration of therapeutic drug candidates, we developed an in vitro neurodegeneration model of ICH, using organotypic brain slice cultures (Fujimoto et al. 2006). Coronal slices containing the cerebral cortex and the striatum were prepared from neonatal rats, and after cultivation for 9–11 days, they were treated with thrombin. Thrombin induced delayed neuronal cell death in the cerebral cortex and tissue shrinkage accompanied by cell death in the striatum. Results of various pharmacological interventions revealed the mechanisms involved in cell and tissue injuries in the cerebral cortex and the striatum. With regard to the tissue injury in the striatum, stimulation of PAR-1 and recruitment of mitogen-activated kinase (MAPK) families plays a critical role. In addition, thrombin-induced striatal tissue injury was abrogated by deletion of tissue microglia prior to thrombin treatment. Importantly, potential involvement of microglial MAPK activation in tissue injury was confirmed in a rat model of ICH induced in the striatum by collagenase injection (Ohnishi et al. 2007), which substantiates the validity of slice culture experiments as a model of ICH pathogenesis.
Then we examined the effect of nicotine on thrombin neurotoxicity in cortico-striatal slice cultures (Ohnishi et al. 2009). In this set of experiments, cortico-striatal slices were prepared and cultured for 11 days in serum-containing medium, maintained for 1 day in serum-free medium, and then treated with 100 U/ml thrombin in serum-free medium for 3 days. When applied to slice cultures for the entire culture period of 15 days, nicotine (3–30 μM) attenuated thrombin-induced shrinkage of striatal tissue and prevented thrombin-induced neuron loss in the cerebral cortex. Thrombin-induced increase in the number of microglia in the cortex was also attenuated by nicotine in a concentration-dependent manner. The cytoprotective effect and anti-inflammatory effect of nicotine were largely abrogated by mecamylamine (non-selective nAChR antagonist), methyllycaconitine (α7 nAChR antagonist) and dihydro-β-erythroidine (α4β2 nAChR antagonist), suggesting the involvement of both of the major central nAChR subtypes.
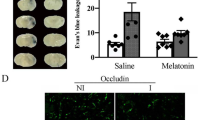
With these lines of evidence in vitro, we moved onto investigations in vivo to address the effect of nicotine in a mouse model of ICH prepared by injection of collagenase (that disrupts vascular basement membrane) into the striatum. Based on the findings in slice culture preparations, we initially examined the effect of long-term pretreatment of nicotine (1 mg/kg, intraperitoneally once per day for 1 week) and obtained cytoprotective effect of nicotine on striatal neurons within the hematoma assessed at 3 days after induction of ICH (our unpublished observations). However, the long-term pretreatment regimen is unrealistic for ICH pharmacotherapy in clinical practice, so we next examined the effect of nicotine in a post-treatment regimen. ICH was induced by collagenase injection into the striatum, and nicotine tartrate dehydrate (1 and 2 mg/kg as nicotine free base) was first administered intraperitoneally 3 h after ICH induction. Nicotine treatment was repeated three times in total, at 24 h intervals. Under these conditions, nicotine (2 mg/kg) partially but significantly attenuated the decrease in the number of striatal neurons in the central region of hematoma at 3 days after ICH. ICH-induced increase in TUNEL-positive cells that may represent apoptotic cell death was also inhibited by nicotine treatment. Interestingly, nicotine decreased expression of a pro-apoptotic protein Bax in the striatum and consequently increased the relative expression level of Bcl-2, an anti-apoptotic protein. Therefore, counteraction of the apoptotic cell death events may underlie the neuroprotective effect of nicotine in ICH (Fig. 7.4). In the peri-hematoma region, the pathological features were accumulation of activated microglia/macrophages and increase in oxidative stress (as revealed by nitrotyrosine immunoreactivity), both of which were significantly attenuated by nicotine treatment. Moreover, nicotine at 2 mg/kg alleviated neurological deficits observed after ICH (Hijioka et al. 2011). When the effect of α7 nAChR agonist PNU-282987 (3 and 10 mg/kg) was tested in the same treatment regimen as nicotine, the drug showed neuroprotective and anti-inflammatory effects, whereas α4β2 nAChR agonist RJR-2403 showed no significant therapeutic effect (Hijioka et al. 2012). These results suggest that α7 nAChR is the major nAChR subtype responsible for the therapeutic effect of nicotine in ICH.
nAChRs expressed in distinct types of cells may contribute to the therapeutic effects of nAChR agonists against brain tissue damage associated with intracerebral hemorrhage (ICH). Stimulation of nAChRs (probably α7 nAChRs) by nicotine in neurons results in decreased expression of a pro-apoptotic protein Bax, thereby inhibits ICH-induced apoptotic neuronal cell death (Hijioka et al. 2011, 2012). On the other hand, specific α7 nAChR agonists such as PNU-282987 and PHA-543613 may stimulate α7 nAChRs in endothelial cells, and recruit Jak2/PI3K/Akt pathway as in the case with Fig. 7.2. Activated Akt phosphorylates and inactivates glycogen synthase kinase-β (GSK-3β) and thereby prevents phosphorylation and degradation of β-catenin, a component of tight junctions. Stabilization of tight junctions may be relevant to the maintenance of integrity of the blood-brain barrier after hemorrhagic insults (Krafft et al. 2013)
Consistent with our findings, Krafft et al. (2012) reported the therapeutic effect of PNU-282987 (12 mg/kg) and another α7 nAChR agonist PHA-543613 (4 or 12 mg/kg) in a mouse model of ICH based on autologous blood infusion into the striatum. In their study, agonists were administered intraperitoneally at 1 h after ICH surgery. These drug treatments improved behavioral outcome and prevented brain edema formation at 24 h after surgery. The effect of PHA-543613 was counteracted by a phosphoinositide 3-kinase (PI3K) inhibitor wortmannin. In addition, PHA-543613 increased the level of phosphorylated Akt and prevented ICH-induced activation of glycogen synthase kinase (GSK) -3β and caspase-3, which was also cancelled by wortmannin. These results indicate that the therapeutic effect of α7 nAChR stimulation is mediated by PI3K/Akt pathway leading to inactivation of GSK-3β and prevention of apoptotic cell death events. A follow-up study by the same group (Krafft et al. 2013) showed that PHA-543613-induced inactivation of GSK-3β led to stabilization of β-catenin and tight junction proteins such as claudin-3 and claudin-5, which may be relevant to the preservation of blood-brain barrier integrity and to the inhibition of edema formation by the drug (Fig. 7.4). A more recent study addressed the role of Jak – signal transducer and activator of transcription (STAT) signaling pathway in the effect of α7 nAChR stimulation (Krafft et al. 2017). That is, α7 nAChR agonist PHA-543613 augmented phosphorylation/activation of Jak2 and STAT3 in a mouse model of ICH by autologous blood infusion, and Jak2 inhibitor AG490 reversed the brain tissue-preserving effect of PHA-543613 in a rat model of ICH based on collagenase injection.
The main focus of the brain region in preclinical ICH studies has been the striatum (the caudate putamen), because the putamen is one of the most susceptible brain regions with regard to hypertension-associated ICH in humans, and also because this region is relatively easy of access by surgical operations in experimental animals. On the other hand, the relative incidence of non-hypertension-associated ICH that occurs in the cerebral cortex (cortical or lobar hemorrhage) has been increasing in recent years, but therapeutic approaches for cortical hemorrhage have been poorly explored. We addressed whether nicotine can provide therapeutic effect in cortical hemorrhage in mice (Anan et al. 2017). Collagenase injection into the parietal cortex induced hemorrhage, which resulted in motor deficits in mice. Daily intraperitoneal administration of nicotine (1 and 2 mg/kg), with the first injection given at 3 h after ICH induction, significantly improved motor performance. Histochemical examinations on brain sections obtained 3 days after ICH demonstrated that nicotine (2 mg/kg) significantly increased the number of surviving neurons in the hematoma and decreased the number of activated microglia/macrophages in the peri-hematoma region. Notably, the neuroprotective effect of nicotine on cortical hemorrhage was blocked by antagonists at α7 nAChRs (methyllycaconitine) and α4β2 nAChRs (dihydro-β-erythroidine). Therefore, the neuroprotective effect of nicotine in cortical hemorrhage may be mediated by both of these two receptor subtypes, unlike in the case with putaminal hemorrhage where α7 nAChR plays a dominant role.
7.4.2 Effects of nAChR Agonists on Subarachnoid Hemorrhage (SAH)
SAH is one of the major kinds of hemorrhagic stroke distinct from ICH. SAH is characterized by extravasation into the subarachnoid space, which mainly results from the rupture of intracranial aneurysm. At present, there is only one experimental study addressing the effect of nAChR agonist on the outcome of SAH. Duris et al. (2011) examined the effect of α7 nAChR agonist PNU-282987 on an endovascular perforation model of SAH in rats, by administering the drug intraperitoneally at 1 h after perforation surgery. They showed that PNU-282987 at 12 mg/kg ameliorated neurological function at 24 and 72 h after SAH induction, and prevented the increase in brain water content at 24 h. As in the case with ICH (Krafft et al. 2012), α7 nAChR stimulation enhanced phosphorylation/activation of Akt and prevented SAH-induced activation of caspase-3, which was abrogated by PI3K inhibitor wortmannin. Therefore, α7 nAChRs may serve as a promising drug target also for SAH therapy, although detailed pharmacological profiles of neuroprotection should be revealed by further investigations.
7.5 Nicotine, Smoking and Stroke: Potential Associations
As nicotine is the major pharmacoactive compound contained in tobacco that can directly modify the functions of the nervous system and the cardiovascular system, potential relationship between smoking and cerebrovascular disorders suggested by various epidemiological investigations may deserve considerations. It should be noted, however, that tobacco smoke contains many (over 4000) kinds of chemicals and the adverse consequences of smoking are not necessarily and solely attributable to the biological actions of nicotine.
With regard to ICH, current smoking is among the modifiable risk factors (An et al. 2017; Ariesen et al. 2003), although smoking (both current use and history of use) may be more strongly associated with ischemic lacunar stroke than with ICH (Kaplan et al. 2014). Race/population-specific influences of smoking on ICH incidence have also been reported. A study by Faigle et al. (2016) showed that smoking was one of the predictors of mortality in white populations, but not black populations, of ICH patients. In addition, meta-analyses of the risk factors for ICH and ischemic stroke suggest that smoking is a risk factor more frequent in ischemic stroke in white population but not in Chinese population (Tsai et al. 2016). In any cases, whether or not nicotine is responsible for the increased risk of ICH associated with smoking is unknown.
Several reports addressed the association of smoking and nicotine exposure with the risk and the outcome of SAH. In a case-control study on population of 18–49 years of age, exposure to nicotine in pharmaceuticals as well as current smoking has been identified as a risk factor of SAH (Broderick et al. 2003). In addition, a retrospective analysis of the size of ruptured intracranial aneurysm of SAH patients demonstrated that the average maximal aneurysm diameter in patients with combined history of hypertension and smoking was significantly smaller than in patients with hypertension only. The latter results suggest that smoking lowers the threshold of aneurysm rupture in combination with hypertension. On the other hand, a systematic review on four independent studies concerning nicotine replacement therapy for tobacco smoking in SAH patients has concluded that the therapy in the first 21 days does not appear to be associated with increased risk of poor functional outcome, death and vasospasm (Turgeon et al. 2017). Actually, there are reports showing better functional outcome (Carandang et al. 2011) and lower risk of death (Seder et al. 2011) in patients with nicotine replacement therapy than those without the therapy. These results imply the beneficial effects of nicotine against SAH pathology in humans, although more detailed and well-designed studies with larger sample size are clearly required for drawing definitive conclusion.
Current smoking has long been known as a strong risk factor for the incidence of ischemic stroke (Hawkins et al. 2002). Increased number of cigarettes smoked per day has been associated with increased risk of ischemic stroke in a study on 15–49 year-old women (Bhat et al. 2008). With regard to the disease outcome, however, “smoker’s paradox” has been reported in ischemic stroke as in the case with myocardial infarction (Ovbiagele and Saver, 2005). For example, a study on 4305 cases of ischemic stroke patients, smoking was associated with lower all-cause in-hospital mortality (Ali et al. 2013). In another study on ischemic stroke patients that received thrombolytic therapy by intravenous tissue plasminogen activator, smokers had better functional outcome in addition to better recanalization and reperfusion, as compared to non-smokers (Kufner et al. 2013). A recent investigation on the potential causes of smoker’s paradox showed that smokers experienced their first ever stroke 11 years younger than non-smokers, which might explain in part the association between smoking and better functional outcome (Hussein et al. 2017). Notably, subgroup analysis in the same study detected favorable outcome of current smokers in women at or older than 65 years (Hussein et al. 2017).
Overall, although smoking may be considered as an important risk factor for the occurrence of stroke episodes, contribution of nicotine to the increased risk is unclear. On the other hand, several lines of evidence indicate that smoking or nicotine treatment (as the replacement therapy) might result in favorable outcome in several particular cases of stroke.
7.6 Conclusion and Future Perspectives
The functional roles of nAChRs in the central nervous system beyond neurotransmission have been increasingly recognized. Neuroprotective and anti-inflammatory effects of nicotine and other nAChR agonists are of particular interest for the development of novel pharmacotherapies against various kinds of disorders associated with neurodegeneration. This chapter summarized preclinical and clinical findings suggesting the relationship between nicotine/nAChRs and principal types of cerebrovascular disorders such as ischemic stroke, ICH and SAH.
Several lines of experimental approaches using in vitro culture system and in vivo disease models indicated the neuroprotective and ant-inflammatory roles of endogenous cholinergic system against pathogenic events in ischemic stroke. These include exacerbation of pathology by nAChR antagonists, as well as amelioration of pathology by AChE inhibitors and positive allosteric modulators of nAChRs. In contrast, studies addressing the effects of nAChR agonists on experimental ischemic injury models gave conflicting results, where some studies reported beneficial effects but others demonstrated deleterious effects. A possible cause of these discrepancies is the different procedures employed for drug administration. Namely, virtually all studies reporting the deleterious effects of nicotine performed continuous delivery by osmotic pumps or repeated daily administration of nicotine at relatively high doses. On the other hand, studies reporting beneficial effects of nicotine and nAChR agonists generally performed daily administration of the drugs for a few times or low-dose administration in the case of long-term treatment. The complex influences of nicotine and nAChR agonists on disease consequences of experimental ischemia models might be relevant to the complicated influences of smoking on the risk and the outcome of ischemic stroke patients that include “smoker’s paradox”.
The number of investigations on hemorrhagic stroke is much smaller than that on ischemic stroke, but distinct lines of experimental evidence are consistent with the idea that nAChRs may serve as a target for ICH therapy. Post-treatment of animal models of striatal (putaminal) hemorrhage with nicotine or α7 nAChR agonists ameliorates neurological outcome and alleviates various neuropathological changes. Involvement of α4β2 nAChRs in addition to α7 nAChRs in neuroprotection is suggested in the case of cortical (lobar) hemorrhage model. Although smoking has been reported as a risk factor for mortality in particular populations of ICH patients, its relationship with nicotine is not evident. Accordingly, nicotine and subtype-specific nAChR agonists may be considered as novel therapeutic drugs for ICH. This proposal is particularly important because no effective pharmacotherapies for ICH directly aiming at brain tissue preservation have been established to date.
There is only one experimental study addressing the effect of nAChR agonist in SAH model, but according to the results, α7 nAChRs may serve as a promising target for therapy also for SAH. Interestingly, nicotine replacement therapy may be safely applicable to, and might produce beneficial outcome in, smokers after SAH.
An important concern at present is the shortage of information regarding the cellular and molecular mechanisms of actions of nicotine and nAChR agonists under stroke conditions, although a few studies have proposed potential involvement of several signaling pathways such as PI3K/Akt and Jak/STAT. Elucidation of the detailed mechanisms of neuroprotective and anti-inflammatory actions, in conjunction with determination of the ideal drug treatment regimens, may pave the way for developing novel strategies for treatment of distinct types of cerebrovascular disorders, by targeting nAChRs and nAChR-mediated neurotransmission.
References
Akaike A, Takada-Takatori Y, Kume T, Izumi Y (2010) Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of α4 and α7 receptors in neuroprotection. J Mol Neurosci 40:211–216. https://doi.org/10.1007/s12031-009-9236-1
Ali SF, Smith EE, Bhatt DL, Fonarow GC, Schwamm LH (2013) Paradoxical association of smoking with in-hospital mortality among patients admitted with acute ischemic stroke. J Am Heart Assoc 2:e000171. https://doi.org/10.1161/JAHA.113.000171
An SJ, Kim TJ, Yoon BW (2017) Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. Stroke 19:3–10. https://doi.org/10.5853/jos.2016.00864
Anan J, Hijioka M, Kurauchi Y, Hisatsune A, Seki T, Katsuki H (2017) Cortical hemorrhage-associated neurological deficits and tissue damage in mice are ameliorated by therapeutic treatment with nicotine. J Neurosci Res 95:1838–1849. https://doi.org/10.1002/jnr.24016
Ariesen MJ1, Claus SP, Rinkel GJ, Algra A (2003) Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke 34:2060–2065
Bhat VM, Cole JW, Sorkin JD, Wozniak MA, Malarcher AM, Giles WH, Stern BJ, Kittner SJ (2008) Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke 39:2439–2443. https://doi.org/10.1161/STROKEAHA.107.510073
Bradford ST, Stamatovic SM, Dondeti RS, Keep RF, Andjelkovic AV (2011) Nicotine aggravates the brain postischemic inflammatory response. Am J Physiol Heart Circ Physiol 300:H1518–H1529. https://doi.org/10.1152/ajpheart.00928.2010
Broderick JP, Viscoli CM, Brott T, Kernan WN, Brass LM, Feldmann E, Morgenstern LB, Wilterdink JL, Horwitz RI, Hemorrhagic Stroke Project Investigators (2003) Major risk factors for aneurysmal subarachnoid hemorrhage in the young are modifiable. Stroke 34:1375–1381
Carandang RA, Barton B, Rordorf GA, Ogilvy CS, Sims JR (2011) Nicotine replacement therapy after subarachnoid hemorrhage is not associated with increased vasospasm. Stroke 42:3080–3086. https://doi.org/10.1161/STROKEAHA.111.620955
Catanese L, Tarsia J, Fisher M (2017) Acute ischemic stroke therapy overview. Circ Res 120:541–558. https://doi.org/10.1161/CIRCRESAHA.116.309278
Chen Y, Nie H, Tian L, Tong L, Yang L, Lao N, Dong H, Sang H, Xiong L (2013) Nicotine-induced neuroprotection against ischemic injury involves activation of endocannabinoid system in rats. Neurochem Res 38:364–370. https://doi.org/10.1007/s11064-012-0927-6
Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH (2011) α7 nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke 42:3530–3536. https://doi.org/10.1161/STROKEAHA.111.619965
Egea J, Rosa AO, Sobrado M, Gandía L, López MG, García AG (2007) Neuroprotection afforded by nicotine against oxygen and glucose deprivation in hippocampal slices is lost in α7 nicotinic receptor knockout mice. Neuroscience 145:866–872
Egea J, Martín-de-Saavedra MD, Parada E, Romero A, Del Barrio L, Rosa AO, García AG, López MG (2012) Galantamine elicits neuroprotection by inhibiting iNOS, NADPH oxidase and ROS in hippocampal slices stressed with anoxia/reoxygenation. Neuropharmacology 62:1082–1090. https://doi.org/10.1016/j.neuropharm.2011.10.022
Faigle R, Marsh EB, Llinas RH, Urrutia VC, Gottesman RF (2016) Race-specific predictors of mortality in intracerebral hemorrhage: differential impacts of intraventricular hemorrhage and age among blacks and whites. J Am Heart Assoc. https://doi.org/10.1161/JAHA.116.003540
Fujiki M, Kobayashi H, Uchida S, Inoue R, Ishii K (2005) Neuroprotective effect of donepezil, a nicotinic acetylcholine-receptor activator, on cerebral infarction in rats. Brain Res 1043:236–241
Fujimoto S, Katsuki H, Kume T, Akaike A (2006) Thrombin-induced delayed injury involves multiple and distinct signaling pathways in the cerebral cortex and the striatum in organotypic slice cultures. Neurobiol Dis 22:130–142
Furukawa S, Sameshima H, Yang L, Ikenoue T (2013) Activation of acetylcholine receptors and microglia in hypoxic-ischemic brain damage in newborn rats. Brain Dev 35:607–613. https://doi.org/10.1016/j.braindev.2012.10.006
Gonzalez CL, Gharbawie OA, Kolb B (2006) Chronic low-dose administration of nicotine facilitates recovery and synaptic change after focal ischemia in rats. Neuropharmacology 50:777–787
Guan YZ, Jin XD, Guan LX, Yan HC, Wang P, Gong Z, Li SJ, Cao X, Xing YL, Gao TM (2015) Nicotine inhibits microglial proliferation and is neuroprotective in global ischemia rats. Mol Neurobiol 51:1480–1488. https://doi.org/10.1007/s12035-014-8825-3
Han Z, Li L, Wang L, Degos V, Maze M, Su H (2014a) Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J Neurochem 131:498–508. https://doi.org/10.1111/jnc.12817
Han Z, Shen F, He Y, Degos V, Camus M, Maze M, Young WL, Su H (2014b) Activation of α-7 nicotinic acetylcholine receptor reduces ischemic stroke injury through reduction of pro-inflammatory macrophages and oxidative stress. PLoS One 9:e105711. https://doi.org/10.1371/journal.pone.0105711
Hawkins BT, Brown RC, Davis TP (2002) Smoking and ischemic stroke: a role for nicotine? Trends Pharmacol Sci 23:78–82
Hejmadi MV, Dajas-Bailador F, Barns SM, Jones B, Wonnacott S (2003) Neuroprotection by nicotine against hypoxia-induced apoptosis in cortical cultures involves activation of multiple nicotinic acetylcholine receptor subtypes. Mol Cell Neurosci 24:779–786
Hijioka M, Matsushita H, Hisatsune A, Isohama Y, Katsuki H (2011) Therapeutic effect of nicotine in a mouse model of intracerebral hemorrhage. J Pharmacol Exp Ther 338:741–749. https://doi.org/10.1124/jpet.111.182519
Hijioka M, Matsushita H, Ishibashi H, Hisatsune A, Isohama Y, Katsuki H (2012) α7 nicotinic acetylcholine receptor agonist attenuates neuropathological changes associated with intracerebral hemorrhage in mice. Neuroscience 222:10–19. https://doi.org/10.1016/j.neuroscience.2012.07.024
Hussein HM, Niemann N, Parker ED, Qureshi AI, Collaborators VISTA (2017) Searching for the smoker’s paradox in acute stroke patients treated with intravenous thrombolysis. Nicotine Tob Res 19:871–876. https://doi.org/10.1093/ntr/ntx020
Jiang Y, Li L, Liu B, Zhang Y, Chen Q, Li C (2014) Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS One 9:e102342. https://doi.org/10.1371/journal.pone.0102342
Kagitani F, Uchida S, Hotta H, Sato A (2000) Effects of nicotine on blood flow and delayed neuronal death following intermittent transient ischemia in rat hippocampus. Jpn J Physiol 50:585–595
Kalappa BI, Sun F, Johnson SR, Jin K, Uteshev VV (2013) A positive allosteric modulator of α7 nAChRs augments neuroprotective effects of endogenous nicotinic agonists in cerebral ischaemia. Br J Pharmacol 169:1862–1878. https://doi.org/10.1111/bph.12247
Kaplan EH, Gottesman RF, Llinas RH, Marsh EB (2014) The association between specific substances of abuse and subcortical intracerebral hemorrhage versus ischemic lacunar infarction. Front Neurol 5:174
Katsuki H (2010) Exploring neuroprotective drug therapies for intracerebral hemorrhage. J Pharmacol Sci 114(4):366–378
Kitaoka T, Hua Y, Xi G, Hoff JT, Keep RF (2002) Delayed argatroban treatment reduces edema in a rat model of intracerebral hemorrhage. Stroke 33:3012–3018
Krafft PR, Altay O, Rolland WB, Duris K, Lekic T, Tang J, Zhang JH (2012) α7 nicotinic acetylcholine receptor agonism confers neuroprotection through GSK-3β inhibition in a mouse model of intracerebral hemorrhage. Stroke 43:844–850. https://doi.org/10.1161/STROKEAHA.111.639989
Krafft PR, Caner B, Klebe D, Rolland WB, Tang J, Zhang JH (2013) PHA-543613 preserves blood-brain barrier integrity after intracerebral hemorrhage in mice. Stroke 44:1743–1747. https://doi.org/10.1161/STROKEAHA.111.000427
Krafft PR, McBride D, Rolland WB, Lekic T, Flores JJ, Zhang JH (2017) α7 nicotinic acetylcholine receptor stimulation attenuates neuroinflammation through JAK2-STAT3 activation in murine models of intracerebral hemorrhage. Biomed Res Int 2017:8134653. https://doi.org/10.1155/2017/8134653
Kufner A, Nolte CH, Galinovic I, Brunecker P, Kufner GM, Endres M, Fiebach JB, Ebinger M (2013) Smoking-thrombolysis paradox: recanalization and reperfusion rates after intravenous tissue plasminogen activator in smokers with ischemic stroke. Stroke 44:407–413. https://doi.org/10.1161/STROKEAHA.112.662148
Li Y, Xiao D, Dasgupta C, Xiong F, Tong W, Yang S, Zhang L (2012) Perinatal nicotine exposure increases vulnerability of hypoxic-ischemic brain injury in neonatal rats: role of angiotensin II receptors. Stroke 43:2483–2490. https://doi.org/10.1161/STROKEAHA.112.664698
Li C, Sun H, Arrick DM, Mayhan WG (2016) Chronic nicotine exposure exacerbates transient focal cerebral ischemia-induced brain injury. J Appl Physiol 120:328–333. https://doi.org/10.1152/japplphysiol.00663.2015
Lim DH, Alaverdashvili M, Whishaw IQ (2009) Nicotine does not improve recovery from learned nonuse nor enhance constraint-induced therapy after motor cortex stroke in the rat. Behav Brain Res 198:411–419. https://doi.org/10.1016/j.bbr.2008.11.038
Lorrio S, Sobrado M, Arias E, Roda JM, García AG, López MG (2007) Galantamine postischemia provides neuroprotection and memory recovery against transient global cerebral ischemia in gerbils. J Pharmacol Exp Ther 322:591–599
Martín A, Szczupak B, Gómez-Vallejo V, Domercq M, Cano A, Padro D, Muñoz C, Higuchi M, Matute C, Llop J (2015) In vivo PET imaging of the α4β2 nicotinic acetylcholine receptor as a marker for brain inflammation after cerebral ischemia. J Neurosci 35:5998–6009. https://doi.org/10.1523/JNEUROSCI.3670-14.2015
Mudo G, Belluardo N, Fuxe K (2007) Nicotinic receptor agonists as neuroprotective/neurotrophic drugs. Progress in molecular mechanisms. J Neural Transm (Vienna) 114:135–147
Nagatsuna T, Nomura S, Suehiro E, Fujisawa H, Koizumi H, Suzuki M (2005) Systemic administration of argatroban reduces secondary brain damage in a rat model of intracerebral hemorrhage: histopathological assessment. Cerebrovasc Dis 19:192–200
Nanri M, Miyake H, Murakami Y, Matsumoto K, Watanabe H (1998a) GTS-21, a nicotinic agonist, attenuates multiple infarctions and cognitive deficit caused by permanent occlusion of bilateral common carotid arteries in rats. Jpn J Pharmacol 78:463–469
Nanri M, Yamamoto J, Miyake H, Watanabe H (1998b) Protective effect of GTS-21, a novel nicotinic receptor agonist, on delayed neuronal death induced by ischemia in gerbils. Jpn J Pharmacol 76:23–29
Ohnishi M, Katsuki H, Fujimoto S, Takagi M, Kume T, Akaike A (2007) Involvement of thrombin and mitogen-activated protein kinase pathways in hemorrhagic brain injury. Exp Neurol 206:43–52
Ohnishi M, Katsuki H, Takagi M, Kume T, Akaike A (2009) Long-term treatment with nicotine suppresses neurotoxicity of, and microglial activation by, thrombin in cortico-striatal slice cultures. Eur J Pharmacol 602:288–293. https://doi.org/10.1016/j.ejphar.2008.11.041
Ovbiagele B, Saver JL (2005) The smoking-thrombolysis paradox and acute ischemic stroke. Neurology 65:293–295
Parada E, Egea J, Romero A, del Barrio L, García AG, López MG (2010) Poststress treatment with PNU282987 can rescue SH-SY5Y cells undergoing apoptosis via α7 nicotinic receptors linked to a Jak2/Akt/HO-1 signaling pathway. Free Radic Biol Med 49:1815–1821. https://doi.org/10.1016/j.freeradbiomed.2010.09.017
Parada E, Egea J, Buendia I, Negredo P, Cunha AC, Cardoso S, Soares MP, López MG (2013) The microglial α7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid Redox Signal 19:1135–1148. https://doi.org/10.1089/ars.2012.4671
Paulson JR, Roder KE, McAfee G, Allen DD, Van der Schyf CJ, Abbruscato TJ (2006) Tobacco smoke chemicals attenuate brain-to-blood potassium transport mediated by the Na, K, 2Cl-cotransporter during hypoxia-reoxygenation. J Pharmacol Exp Ther 316:248–254
Paulson JR, Yang T, Selvaraj PK, Mdzinarishvili A, Van der Schyf CJ, Klein J, Bickel U, Abbruscato TJ (2010) Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. J Pharmacol Exp Ther 332:371–379. https://doi.org/10.1124/jpet.109.157776
Qureshi AI, Mendelow AD, Hanley DF (2009) Intracerebral hemorrhage. Lancet 373:1632–1644. https://doi.org/10.1016/S0140-6736(09)60371-8
Raval AP, Bhatt A, Saul I (2009) Chronic nicotine exposure inhibits 17β-estradiol-mediated protection of the hippocampal CA1 region against cerebral ischemia in female rats. Neurosci Lett 458:65–69. https://doi.org/10.1016/j.neulet.2009.04.021
Raval AP, Hirsch N, Dave KR, Yavagal DR, Bramlett H, Saul I (2011) Nicotine and estrogen synergistically exacerbate cerebral ischemic injury. Neuroscience 181:216–225. https://doi.org/10.1016/j.neuroscience.2011.02.036
Ray RS, Rai S, Katyal A (2014) Cholinergic receptor blockade by scopolamine and mecamylamine exacerbates global cerebral ischemia induced memory dysfunction in C57BL/6J mice. Nitric Oxide 43:62–73. https://doi.org/10.1016/j.niox.2014.08.009
Seder DB, Schmidt JM, Badjatia N, Fernandez L, Rincon F, Claassen J, Gordon E, Carrera E, Kurtz P, Lee K, Connolly ES, Mayer SA (2011) Transdermal nicotine replacement therapy in cigarette smokers with acute subarachnoid hemorrhage. Neurocrit Care 14:77–83
Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J (2004) Cholinergic modulation of microglial activation by α7 nicotinic receptors. J Neurochem 89:337–343
Sun Z, Baker W, Hiraki T, Greenberg JH (2012) The effect of right vagus nerve stimulation on focal cerebral ischemia: an experimental study in the rat. Brain Stimul 5(1):1–10
Sun F, Jin K, Uteshev VV (2013) A type-II positive allosteric modulator of α7 nAChRs reduces brain injury and improves neurological function after focal cerebral ischemia in rats. PLoS One 8:e73581. https://doi.org/10.1371/journal.pone.0073581
Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP (2009) Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov 8:733–750. https://doi.org/10.1038/nrd2927
Tsai CF, Anderson N, Thomas B, Sudlow CL (2016) Comparing risk factor profiles between intracerebral hemorrhage and ischemic stroke in Chinese and white populations: systematic review and meta-analysis. PLoS One 11:e0151743. https://doi.org/10.1371/journal.pone.0151743
Turgeon RD, Chang SJ, Dandurand C, Gooderham PA, Hunt C (2017) Nicotine replacement therapy in patients with aneurysmal subarachnoid hemorrhage: systematic review of the literature, and survey of Canadian practice. J Clin Neurosci 42:48–53. https://doi.org/10.1016/j.jocn.2017.03.014
Wang L, Kittaka M, Sun N, Schreiber SS, Zlokovic BV (1997) Chronic nicotine treatment enhances focal ischemic brain injury and depletes free pool of brain microvascular tissue plasminogen activator in rats. J Cereb Blood Flow Metab 17:136–146
Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ (2003) Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421:384–388
Wang ZF, Wang J, Zhang HY, Tang XC (2008) Huperzine A exhibits anti-inflammatory and neuroprotective effects in a rat model of transient focal cerebral ischemia. J Neurochem 106:1594–1603. https://doi.org/10.1111/j.1471-4159.2008.05504.x
Zoli M, Pistillo F, Gotti C (2015) Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 96:302–311. https://doi.org/10.1016/j.neuropharm.2014.11.003
Acknowledgments
This work was supported by the Smoking Researh Foundation and JSPS KAKENHI, MEXT, Japan (Grants 16H04673 and 16 K15204).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Katsuki, H., Matsumoto, K. (2018). Nicotinic Acetylcholine Receptors in Regulation of Pathology of Cerebrovascular Disorders. In: Akaike, A., Shimohama, S., Misu, Y. (eds) Nicotinic Acetylcholine Receptor Signaling in Neuroprotection. Springer, Singapore. https://doi.org/10.1007/978-981-10-8488-1_7
Download citation
DOI: https://doi.org/10.1007/978-981-10-8488-1_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8487-4
Online ISBN: 978-981-10-8488-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)