Abstract
Campylobacter jejuni is the principal bacterial foodborne pathogen. A major challenge still is to identify the virulence strategies exploited by C. jejuni. Recent genomics, proteomics, and metabolomics approaches indicate that C. jejuni displays extensive inter- and intrastrain variation. The diverse behavior enables bacterial adaptation to different environmental conditions and directs interactions with the gut mucosa. Here, we report recent progress in understanding the molecular mechanisms and functional consequences of the phenotype diversity. The results suggest that C. jejuni actively penetrates the intestinal mucus layer, secretes proteins mainly via its flagellar apparatus, is engulfed by intestinal cells, and can disrupt the integrity of the epithelial lining. C. jejuni stimulates the proinflammatory pathway and the production of a large repertoire of cytokines, chemokines, and innate effector molecules. Novel experimental infection models suggest that the activation of the innate immune response is important for the development of intestinal pathology.
Similar content being viewed by others
Keywords
- Miller Fisher Syndrome
- Intestinal Pathology
- Haemophilus Ducreyi
- Bacterial Foodborne Pathogen
- Horizontal Gene Exchange
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Campylobacter jejuni is estimated to cause approximately 400 million cases of human enterocolitis per year. In developing countries, Campylobacter is the most commonly isolated bacterial pathogen from young children with diarrhea (Coker et al. 2002). At older ages, most infections are usually mild or asymptomatic, probably because of immunity that may follow frequent exposure to contaminated food or water (Allos and Blaser 1995; Havelaar et al. 2009). In industrialized nations, C. jejuni is the leading bacterial foodborne pathogen and one of the most important causative agents of traveler’s diarrhea. Ingestion of as few as 500 bacteria is sufficient to develop symptomatic disease (Black et al. 1988). The bacteria colonize the distal small intestine and the colon, and induce mucosal edema, cellular infiltrates, small abscesses, and focal ulcerations (Colgan et al. 1980). Clinical manifestations are fever, abdominal cramps, and bloody or watery diarrhea (Allos and Blaser 1995). Although the symptoms generally resolve within 5–7 days, the economical burden caused by C. jejuni is estimated to be up to 8 billion dollars per year in the US alone (Buzby and Roberts 1997). This is partly attributed to severe complications that can follow C. jejuni infection, such as reactive arthritis and the paralyzing autoimmune neuropathies, Guillain-Barré syndrome and Miller Fisher syndrome (Yuki et al. 2004). The association between C. jejuni infection and the occurrence of irritable bowel syndrome (Spiller 2007) and immunoproliferative intestinal lymphomas (IPSID) (Lecuit et al. 2004) is still under investigation.
In comparison to other intestinal pathogens of global importance, C. jejuni pathogenesis is still poorly understood. Recent genomics, proteomics, and advanced infection biology approaches, however, have led to the discovery of important bacterial traits including the presence of a polysaccharide capsule and sophisticated protein glycosylation machineries. Furthermore, metabolic adaptation in response to changing environments, flagella-driven motility, chemotaxis, protein secretion, colonization of mucus, bacterial infection of mucosal cells, and toxin production appear key steps in the establishment of infection. Here, we will discuss the state-of-the-art of the molecular pathogenesis of C. jejuni infection of the gut.
2 C. jejuni Genetics and Diversity
2.1 Genome Variation
C. jejuni is a Gram-negative spiral-shaped bacterium that needs a microaerophilic growth environment of 30–44°C under laboratory conditions. The bacterium is highly motile due to the presence of a single flagellum at each pole. The C. jejuni genome is relatively small (1.6–1.7 Mb) (Parkhill et al. 2000), but shows considerable genetic diversity among individual C. jejuni isolates. Horizontal gene exchange and natural competence for DNA uptake likely contribute to the largely nonclonal nature of the species. Genome-wide analysis of multiple C. jejuni isolates suggests that about 20% of the C. jejuni genome varies between strains with the presence of unique sets of genes in different isolates (Pearson et al. 2003; Poly et al. 2005; Hofreuter et al. 2006). Approximately 50% of the variable gene pool is located in hypervariable loci involved in the biosynthesis and posttranslational modification of flagellin, in capsule and lipo-oligosaccharide (LOS) production, and in DNA restriction/modification. A relatively large number of C. jejuni genes within the variable DNA regions contain tracts of repetitive nucleotide repeats (Wassenaar et al. 2002; Parkhill et al. 2000). These homopolymeric tracts are prone to undergo high rate slipped-strand mispairing resulting in high frequency on-off switching of gene function.
The C. jejuni genome does not contain typical pathogenicity islands. However, individual strains may contain one or more C. jejuni-integrated elements (CJIEs) with phage characteristics (Fouts et al. 2005; Parker et al. 2006; Clark and Ng 2008) and/or different types of cryptic plasmids (Miller et al. 2007). Conjugative plasmids are frequently found, but generally poorly characterized. A small subset of isolates carries the pVir plasmid (Bacon et al. 2002; Tracz et al. 2005). This plasmid contains elements of a putative type IV secretion system that in other bacterial species is involved in DNA export, conjugation, and protein secretion. In C. jejuni, the pVir plasmid is not required to establish an infection and does not appear to be associated with the development of bloody diarrhea (Louwen et al. 2006) in contrast to earlier suggestions (Tracz et al. 2005).
2.2 Phenotype Diversity in C. jejuni
The variable gene content in C. jejuni isolates generates differences in bacterial phenotype and adaptation potential. At the metabolic level, this is nicely exemplified by the variable ability of strains to utilize glutamine and asparagine as nutrients due to variable presence or allelic variation in the genes encoding gamma-glutamyltranspeptidase (GGT) and a periplasmic asparaginase, respectively (Hofreuter et al. 2008). This may influence bacterial colonization (Barnes et al. 2007). Similarly, strains may secrete isoforms of the FspA protein that differ in their ability to induce host cell apoptosis (Poly et al. 2007), while variable presence of CJIEs contributes to the difference in natural transformability between C. jejuni strains due to encoded DNase activity (Gaasbeek et al 2009). Strain variations in the composition of the flagellar locus can lead to both differences in the flagellin protein backbone and in variable post-translational modifications of flagellin. This variation influences antigenicity and flagella function, i.e., autoagglutination behavior. Even more marked strain diversity originates from the variable composition of the capsular and LOS biosynthesis loci. This results in the presence of many capsule types (Karlyshev et al. 2005) and a huge repertoire of produced surface lipo-oligosaccharides (Karlyshev et al. 2005; Parker et al. 2008). The capsule surface variation is often accompanied by a change in different antigenic properties (Karlyshev et al. 2005) and may contribute to the variable susceptibility of C. jejuni to bacteriophages (Coward et al. 2006). The clinical importance of the LOS diversity is illustrated through the association between the distinct LOS glycoforms that mimic host cell gangliosides and the development of Guillain–Barré syndrome (Yuki et al. 2004). The LOS structures may also differentially interact with host lectin receptors and thus influence the pathogen–host interaction. Thus far, phenotype diversity of C. jejuni is rarely taken into account in molecular pathogenesis studies, most of which are performed with a limited set of strains (e.g., strains 11168, 81–176, and 81116).
2.3 Intrastrain Phenotype Variation
In addition to differences in gene content that may explain diversity in behavior between C. jejuni isolates, individual strains display extensive phenotype variation. Two major mechanisms contribute to the intrastrain phenotype diversity, genetic variation and gene regulation. The genetic variation is largely based on the large number of homopolymeric DNA repeats in the genome. This often leads to uncontrolled variation in promoter activity or a shift in open reading frames. The seemingly random on-and-off switching of genes in the population yields a bacterial progeny that is heterogeneous in the production and/or structure of major surface components including the capsular polysaccharide, LOS and flagellin (Bacon et al. 2001; Linton et al. 2000; Guerry et al. 2002; van Alphen et al. 2008b). This diversity can be highly beneficial to the C. jejuni isolate as a heterogeneous set of bacterial phenotypes can prepare the bacterial population to survive changing environmental conditions.
Apart from via (random) genetic variation, the C. jejuni can switch phenotype by controlled regulation of gene expression. This type of regulation usually acts at the level of the entire population rather than of individual bacteria and typically occurs in response to distinct environmental cues. Illustrative examples are the availability of iron and phosphate, which regulate the biosynthesis of iron and phosphate acquisition systems (Palyada et al. 2004; Wösten et al. 2006). Other traits of C. jejuni that appear to be subject to gene regulation are capsule production, flagella synthesis, flagella-mediated protein secretion, and biofilm formation. The molecular mechanisms that drive these events and their importance for C. jejuni colonization and virulence largely remain to be determined.
2.4 Metabolic Adaptation
C. jejuni encounters a variety of environmental niches ranging from surface water to the gut of animals and humans. Survival under these conditions requires intricate adaptation machineries that enable C. jejuni to switch between, e.g., different nutrient sources, and to respond to alterations in oxygen availability and temperature such as exist in the intestine of different hosts. Microarray analysis of C. jejuni cultured under different environmental conditions demonstrates major differences in gene expression after growth at 37 and 42°C, simulating the body temperatures of human and chicken, respectively (Stintzi 2003). Similarly, in a limited oxygen environment, C. jejuni can switch to alternative electron acceptors including fumarate and nitrite (Sellars et al. 2002), and utilize alternative amino acids as preferred carbon source (Guccione et al. 2008; Wright et al. 2009). The change in metabolic state in different environmental niches may alter C. jejuni virulence properties. In chickens, which are a major reservoir of C. jejuni, colonization of the cecum occurs without apparent intestinal pathology. The bacteria preferentially reside in the mucus in close proximity to the epithelial cells but apparently do not adhere to or invade the intestinal tissue (Beery et al. 1988; Meinersmann et al. 1991). The altered body temperature and the much more abundant presence in the chicken cecum of the amino acids serine, proline, aspartate, and glutamate, that are preferentially metabolized by C. jejuni, may influence C. jejuni behavior such as bacterial growth and chemotaxis and thereby alter bacterial virulence.
The environmental changes in C. jejuni behavior appear mainly driven via sophisticated two-component signal transduction systems that control the expression of distinct metabolic regulons (reviewed in Wösten et al. 2008) and posttranscriptional regulatory mechanisms (Yun et al. 2008; Fields and Thompson 2008). Infection experiments in chickens with C. jejuni with genetically defined defects in different two-component signal transduction systems demonstrate that they are essential for bacterial colonization and/or persistence in the intestine (Brás and Ketley 1999; Svensson et al. 2009; MacKichan et al. 2004; Wösten et al. 2004). Future expression profiling of human C. jejuni intestinal isolates may reveal which adaptation machineries are activated in the human intestine and whether these systems affect C. jejuni virulence traits.
3 C. jejuni Virulence Repertoire
3.1 Campylobacter Infection in Humans
Human volunteer studies using clinical isolates confirm that C. jejuni causes dysenteric symptoms with high numbers of leukocytes in the feces (Black et al. 1988). Pathology on intestinal biopsies and experimental animal models show damage to columnar epithelial cells, increased exfoliation, necrosis, diffuse neutrophil infiltration of the lamina propria with superficial crypt abscesses, and histopathological features similar to Salmonella- and Shigella-induced colitis (Black et al. 1988; Russell et al. 1989). C. jejuni is also found inside colonic mucosal cells (van Spreeuwel et al. 1985), indicating that Campylobacter is able to invade human epithelial cells in vivo. The molecular basis of C. jejuni intestinal pathology is not completely understood. The major bacterial traits that are thought to contribute to C. jejuni colonization and pathogenesis are outlined below.
3.2 Flagella and Flagella-Mediated Motility
Flagella-mediated motility is highly important for the successful C. jejuni colonization of the gastrointestinal tract of experimental animals and human volunteers (Morooka et al. 1985; Walker et al. 1986). Bacterial motility is conferred through a single unsheathed flagellum that is present at each pole. Over 40 genes are involved in C. jejuni flagella biogenesis and assembly (Wösten et al. 2004, 2008). C. jejuni produces two different (approximately 59-kDa) flagellin subunits, FlaA and FlaB, that are both incorporated into the flagellum although not in equal amounts. The expression of FlaA and FlaB is controlled by different transcription factors, namely the alternative sigma28 (FlaA) and sigma54 (FlaB) transcription factors (Wösten et al. 2004; Hendrixson and DiRita 2003). The biological advantage of the differential regulation of the expression of the two flagellins is unknown. Both types of flagellin can be assembled into a filament structure and differ in only 9–10 amino acids in their otherwise conserved domains that form the backbone of the flagella fiber. It cannot be excluded that the amino acid differences influence the properties and/or structure of the hollow fiber or perhaps the protein secretion through the filament, but this awaits further study.
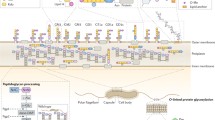
Flagella assembly in C. jejuni is unusual as the filament consists of 7 rather than 11 protofilaments (Galkin et al. 2008) and because flagella formation requires glycosylation of the flagellin monomers (Linton et al. 2000; Karlyshev et al. 2002; Goon et al. 2003) (Fig. 1). The molecular basis for the altered architecture of the flagellum and the need for glycosylation are unknown. The altered packaging of the flagellum may have co-evolved with the inability of C. jejuni flagellins to activate the innate Toll-like receptor TLR5 (Galkin et al. 2008). The glycosylation of the flagellins may enforce structural requirements important in the export and/or polymerization of flagellins, although this would not explain the existing variation in glycans used to decorate the flagellins. Alternatively, the sugar coat on the flagellum may provide strength, rigidity, and charge that facilitate the C. jejuni to access more viscous environment.
Schematic structure of the flagellar secretion apparatus and the different types of carbohydrates structures of C. jejuni. The flagellum is composed of a basal body embedded in the membrane and an extracellular fiber consisting of thousands of flagellin polymers. The C. jejuni flagellins are decorated with variable O-linked carbohydrates. Other variable carbohydrate surface structures are the outer membrane lipooligosaccharide (LOS) and the polysaccharide capsule (CPS). The largely conserved N-linked glycans are attached mainly to proteins in the periplasm. In the absence of a type III secretion system, the flagellar secretion apparatus appears to secrete several putative virulence proteins including FspA, FlaC and up to eight Cia proteins
The composition in the surface-exposed regions of both FlaA and FlaB is highly variable among isolates due to horizontal exchange and recombinatorial events (Wassenaar et al. 1995) and heterogeneity of the attached glycan moieties. The glyco-modifications are encoded by the O-glycosylation locus (Guerry et al. 2006). This locus varies in gene composition between strains and contains several contingency genes that contribute to intrastrain carbohydrate heterogeneity (Guerry et al. 2006). State-of-the-art chemical analysis indicate that the attached carbohydrates predominantly consist of pseudaminic acid or legionaminic acid derivatives that are attached to distinct serine and threonine residues in the variable domain (Thibault et al. 2001; Logan et al. 2002, 2009; McNally et al. 2006; Guerry and Szymanski 2008). Variable substitution of the acetamido group of pseudaminic acid with acetaminido or hydroxyproprionyl groups causes further microheterogeneity in the carbohydrates moieties (Thibault et al. 2001). While the variation in amino acid sequence of the variable domain does not seem to influence flagella-mediated motility, the attached glycans do influence flagella-mediated auto-agglutination and microcolony formation (Guerry 2007; Guerry et al. 2006; van Alphen et al. 2008b) and thus are functionally relevant. The diversity in the surface-exposed region of C. jejuni flagellins may also serve to escape attack from the immune system (Andersen-Nissen et al. 2005) and bacteriophages (Coward et al. 2006).
3.3 Chemotaxis
With their flagella, C. jejuni can specifically swim towards a variety of substances or away from harmful environments. The directed motility appears to be driven by at least two types of taxis systems, namely chemotaxis that responds to environmental stimuli, and energy-taxis that drives motility towards environments promoting optimal electron transport. Major chemoattractants include mucin and the amino acids serine, proline, aspartate, glutamate (Hugdahl et al. 1988). Identified activators of the C. jejuni energy taxis system are fumarate and pyruvate, which yield high levels of electron transport and ATP (Hendrixson et al. 2001). In silico analysis of the C. jejuni genome sequence predicts the presence of at least nine putative chemotaxis sensing receptors (methyl accepting receptors, MCPs), the energy-taxis proteins CetA and CetB, one chemotaxis protein with potential histidine kinase activity (CheA), and multiple proteins with a CheY-like response regulator domains (CheY, CheA and CheV), as well as CheZ, CheV, CheB and CheR orthologs that may be involved in signal amplification and/or adaptation to chemotactic stimuli (Marchant et al 2002).
At the functional level, C. jejuni CheA and CheY are required for directional motility in soft agar (Golden and Acheson 2002; Hendrixson et al. 2001; Yao et al. 1997) as noted for other bacterial species. A C. jejuni CheY mutant is hyperinvasive in cultured cells (Golden and Acheson 2002; Hickey et al. 1999) possibly because this mutant is still capable of flagella-based movement but is no longer directional (Yao et al. 1997). Both CheY and CheA mutants are deficient in colonization of the mouse or chicken gastrointestinal tract (Takata et al. 1992; Yao et al. 1997; Hendrixson and DiRita 2004), indicating that chemotaxis is essential for colonization.
The energy-taxis system of C. jejuni involves the sensory protein complex CetB and CetA (Hendrixson et al. 2001). The complex shares features with the energy-taxis receptor Aer of E. coli including the presence of domains of Aer (divided between CetA and CetB), a sensory PAS domain in CetB, and a predicted transmembrane region, HAMP domain and HCD domain in CetA. Interestingly, CetA and CetB are co-transcribed independently of the flagellar regulon (Elliott and DiRita 2008). While CetA and CetB mutants display normal colonization of the chicken intestine (Hendrixson and DiRita 2004), inactivation of CetA but not CetB causes a moderate (five-fold) reduction in invasion of cultured human epithelial cells (Elliott et al. 2009). Thus, the energy-taxis system may be important for virulence rather than colonization and/or may display host-specific activation. The signals that activate the C. jejuni CetA/CetB energy-taxis complex in vivo and the molecular basis of the altered invasive properties are still unresolved.
3.4 C. jejuni Capsule and LOS
Like most Gram-negative bacterial species, C. jejuni produces surface glycolipids including capsular polysaccharide (CPS) and lipo-oligosaccharide (LOS) (Fig. 1). The polysaccharide capsule was first discovered after analysis of the C. jejuni genome sequence (Parkhill et al. 2000) and confirmed by electron microscopy (Karlyshev et al. 2001). The capsule consists of repeating oligosaccharide units attached to a dipalmitoyl-glycerophosphate lipid anchor (St Michael et al. 2002; Corcoran et al. 2006). The CPS is extensively substituted with variable O-methyl phosphoramidate, methyl, ethanolamine, and N-glycerol groups (McNally et al. 2007). The structural variation in CPS is consistent with the noted genetic diversity in the cps gene cluster and is the basis of the Penner serotyping used to distinguish C. jejuni isolates (Karlyshev et al. 2005). Environmental regulation of capsule biosynthesis as present in many other bacterial pathogens has not been investigated in great detail. However, capsular biosynthesis undergoes frequent phase variation, suggesting that the C. jejuni capsular phenotype is variable (Bacon et al. 2001). Capsule-deficient C. jejuni show increased surface hydrophobicity and serum sensitivity, and reduced invasion of INT-407 cells and virulence, in ferrets (Bacon et al. 2001).
The majority of the C. jejuni cell wall consists of oligosaccharides attached to a lipid A anchor (LOS). The structure of C. jejuni lipid A follows the same architectural principle as in most other Gram-negative species, although the lipid A backbone of C. jejuni is composed of a phosphorylated disaccharide containing diaminoglucose and glucosamine as the major molecular species with slightly longer N- and O-linked acyl chains (Moran 1995). The disaccharide is variably substituted with phosphate or phosphoethanolamine (Moran 1997). The biological significance of the different core structure is unknown. C. jejuni lipid A seems to have a lower fluidity at 37°C than Salmonella LPS, possibly because of the different acyl chain characteristics. C. jejuni LOS activates the proinflammatory TLR4/MD2 pathway (de Zoete and van Putten, unpublished results), but is less biologically active than Salmonella LPS with regard to toxicity, pyrogenicity, and the induction of TNFα (Moran 1995).
The LOS inner core region consists of two heptose residues attached to one KDO molecule. The distal heptose can contain O-linked glycine (Dzieciatkowska et al. 2007). The outer core of C. jejuni LOS is highly variable in structure. Frequent horizontal gene exchange and rearrangements of LOS genes has resulted in mosaic-like organization of the LOS gene cluster with different strains each have their own LOS gene repertoire (Parker et al. 2008; Gilbert et al. 2002). Additional intrastrain LOS heterogeneity stems from the variable expression of genes with homopolymeric nucleotide tracts that are prone to slipped-strand mispairing (Parkhill et al. 2000). Other mechanisms contributing to the LOS diversity are mutations leading to gene inactivation or different acceptor specificities of glycosyltransferases (Gilbert et al. 2002). Of particular biological importance is the ability of most C. jejuni strains to variably incorporate sialic acids into its LOS (Godschalk et al. 2007; Guerry et al. 2002; St Michael et al. 2002). This can result in the formation of ganglioside-like structures as reported for Helicobacter pylori, Haemophilus influenzae, Haemophilus ducreyi, and the pathogenic Neisseria species. The ganglioside mimics may elicit pathogenic antibodies that cross-react with host cell glycolipids and contribute to the development of the autoimmunity-based Guillain-Barré syndrome (Yuki et al. 2004). C. jejuni LOS may promote adhesion to and invasion of host cells (Fry et al. 2000; Guerry et al. 2002) and may target host cell glycan receptors with immunomodulatory functions (Louwen et al. 2008; Avril et al. 2006). The variable oligosaccharide structure may thus aid C. jejuni to colonize different hosts or intestinal niches.
In addition to capsule and LOS, C. jejuni may produce another type of polysaccharide that may be involved in biofilm formation (Kalmokoff et al. 2006; Joshua et al. 2006). The nature of this surface polysaccharide is unknown but its biosynthesis may require carbamoylphosphate synthase (McLennan et al. 2008). Biofilm formation is upregulated under anaerobic conditions and in a C. jejuni SpoT mutant. This mutant is defective for the stringent response that is important for survival of environmental stress (McLennan et al. 2008). The formation of C. jejuni biofilms may contribute to survival in aquatic environments. Its role in C. jejuni virulence remains to be determined.
3.5 Surface Proteins and Protein Glycosylation
The number of identified surface-exposed membrane proteins in C. jejuni is limited. One principal protein is the major outer membrane porin, MOMP (Moser et al. 1997; Dé et al. 2000). C. jejuni MOMP is a β-barrel protein with surface exposed loops that are hypervariable in amino acid composition between isolates (Clark et al. 2007), suggestive of selective pressure by the immune system. A second porin protein, Omp50, is upregulated during in vivo growth of C. jejuni (Stintzi et al. 2005). Other C. jejuni surface proteins include the fibronectin binding protein CadF (Konkel et al. 1997) and the lipoproteins Omp18 (Burnens et al. 1995; Konkel et al. 1996) and JlpA (Jin et al. 2001). A separate class of proteins are the PEB proteins implicated in amino acid and phosphate transport as well as bacterial adherence to eukaryotic cells (Pei et al. 1998; Leon-Kempis Mdel et al. 2006). How these proteins exert this dual function remains to be established.
C. jejuni produces two putative autotransporters, CapA and CapB (Ashgar et al. 2007). In other pathogens, autotransporters represent an extensive and rapidly growing family of secreted virulence-associated proteins. The identified C. jejuni autotransporters each contain a homopolymeric tract and are therefore predicted to undergo phase variation. Insertional inactivation of CapA results in reduced adhesion and invasion of Caco-2 cells and loss of the ability to colonize chickens, suggesting that the protein may play a role in C. jejuni colonization and virulence (Ashgar et al. 2007).
In addition to the O-glycosylated flagellins, C. jejuni contains at least 35 different N-linked glycoproteins (Young et al. 2002). Genes involved in the N-linked protein glycosylation pathway are encoded by the protein glycosylation (pgl) locus (Szymanski et al. 1999). In contrast to the O-glycosylation locus, this locus is conserved among C. jejuni strains which explains why all N-glycoproteins appear to carry the same heptasaccharide moiety, GalNAc-α1,4-GalNAc-α1,4-(Glcβ1,3)-GalNAc-α1,4-GalNAc-α1,4-GalNAc-α1,3-Bac, where Bac is bacillosamine (Young et al. 2002). The oligosaccharide is attached to asparagine residues that are part of the specific glycosylation consensus sequence, Asp/Glu-Y-Asn-X-Ser/Thr, where Y and X is any amino acid except proline (Kowarik et al. 2006). Important here is that virtually all N-glycosylated proteins appear to be located in the periplasm (Fig. 1). Their limited (if at all) surface exposure in the intact bacterium may explain the conserved nature of the oligosaccharide. The function of the N-linked protein glycosylation is still an enigma. Disruption of the glycosylation pathway reduces C. jejuni adherence and invasion in INT-407 cells and the colonization of the intestinal tracts of animals (Szymanksi et al. 2002; Karlyshev et al. 2004). The molecular basis of the attenuated C. jejuni behavior remains to be defined, but may reflect a general dysfunction of the C. jejuni membrane.
3.6 Secreted Factors
C. jejuni secretes several putative virulence factors into its environment. Most identified factors are secreted through the flagellar secretion apparatus (Fig. 1). Apart from flagellar components, this machinery secretes the FlaC, FspA, and at least eight C. jejuni invasion antigens (Cia). The 26-kDa FlaC protein is predicted to resemble FlaA and FlaB except that it lacks the variable central domain of the flagellins (Song et al. 2004). Structurally, the protein FlaC is not required for flagellum formation or motility, but was shown to bind to HEp-2 cells, both when secreted by the bacteria during in vitro infection of HEp-2 cells and as purified recombinant protein (Song et al. 2004).
The approximately 18-kDa FspA protein is present in C. jejuni in either of two variant forms, FspA1 or FspA2. The FspA proteins display considerable heterogeneity between strains. FspA2, but not FspA1, binds eukaryotic cells and induces apoptosis in epithelial cells (Poly et al. 2007).
In contrast to FlaC and FspA, secretion of the Cia proteins requires contact with host cells or the presence of mucin or serum (Rivera-Amill et al. 2001). Deoxycholate induces the transcription of the ciaB gene, and C. jejuni harvested from Muller-Hinton agar plates supplemented with deoxycholate also secrete Cia proteins (Malik-Kale et al. 2008). The apparent diversity in signals that drive Cia secretion suggests that the secretion event may be related to environmental changes rather than a distinct environmental cue. In some C. jejuni strains (but not strain 129108), inactivation of ciaB prevents secretion of other Cia proteins and blocks bacterial invasion of cultured epithelial cells (Konkel et al. 1999).
Like several other enteropathogens, C. jejuni secretes a cytolethal-distending toxin (CDT) (Smith and Bayles 2006). This toxin is comprised of three subunits, CdtA, CdtB, and CdtC, all of which are essential for toxin activity (Lara-Tejero and Galán 2001). CdtA and CdtC are essential for binding to host cells (Lee et al. 2003), while CdtB internalization by the eukaryotic cells is essential for toxicity (Lara-Tejero and Galán 2001). CdtB, which has DNase I-like activity, is translocated to the nucleus and induces eukaryotic cell cycle arrest in the G2 phase (Whitehouse et al. 1998). Cytotoxicity or cell cycle arrest can be achieved by adding a combination of the three purified toxin proteins to cultured epithelial cells (Lee et al. 2003; Lara-Tejero and Galán 2001). In addition to its cytotoxic effect, CDT also appears to stimulate the proinflammatory NF-kappaB pathway and elicits IL-8 secretion (Hickey et al. 2000; Zheng et al. 2008).
C. jejuni also secretes several nonproteinaceous molecules. One important factor may be the auto-inducer AI-2. This compound influences C. jejuni swarming motility, autoagglutination, biofilm formation, sensitivity to hydrogen peroxide, and the transcription of the cytolethal-distending toxin genes (He et al. 2008). C. jejuni AI-2 production, which is dependent upon luxS, is maximal during mid- to late-exponential growth, but rapidly decreases at high cell concentrations during entry into the stationary growth phase (Quinones et al. 2009).
4 Mucosal Infection by C. jejuni
4.1 Penetration of the Mucus Layer
The first barrier C. jejuni encounters in the gut is the mucus layer (Fig. 2). C. jejuni can effectively penetrate this barrier. Pathology studies frequently identify large numbers of bacteria in the mucus layer and in intestinal crypts without apparent attachment to the microvillus (Beery et al. 1988; Lee et al. 1986). Mucin is a strong chemoattractant for C. jejuni (Hugdahl et al. 1988) and, instead of being trapped and transported out of the intestinal tract, the bacterium freely moves in parallel streams along the mucus strands (Lee et al. 1986; McSweegan et al. 1987). This efficient mobility in mucus may be due to its spiral-shaped morphology and the presence of putative mucin-degrading enzymes. In addition, C. jejuni swims at higher speeds in environments of high rather than low viscosity and under conditions that immobilize conventional rod-shaped bacteria (Ferrero and Lee 1988). In media with high viscosity, C. jejuni shows longer periods of straight swimming with increased velocity followed by pauses, resembling the swimming pattern of spirochetes rather than of other monotrichous bacteria (Shigematsu et al. 1998). Whether the change in swimming mode is related to the observed downregulation of the flagellin (flaA) promoter in viscous conditions (Allen and Griffiths 2001) awaits further study.
Colonization and invasion strategies of C. jejuni. The pathogen can actively swim into the mucus layer and survive in intestinal crypts. Uptake and transport across M cells may enable C. jejuni to migrate into the subcellular environment and to invade epithelial cells at the cell basis. Transient disruption of tight junctions between intestinal cells may enable penetration of the epithelial lining via the paracellular route. Bacteria-directed uptake into epithelial cells may lead to transcellular transport and exocytosis at the cell basis, as well as to trafficking to a unique intracellular compartment in proximity of the Golgi complex. The contribution of the various invasion strategies of C. jejuni to the establishment of a natural infection is unknown
The use of mucus or high-viscosity medium during in vitro infection tends to increase C. jejuni adherence and invasion of epithelial cells. On other hand, mucus of rabbits previously colonized with C. jejuni impede the bacterial adherence to INT-407 cells due to the presence of Campylobacter-specific IgA, which causes aggregation of C. jejuni within the mucus layer (McSweegan et al. 1987). This observation may at least partially explain why frequent C. jejuni exposure protects against intestinal pathology.
4.2 Cellular Infection
After mucus penetration, C. jejuni can come in close contact with the intestinal epithelial cells. Despite excellent work, the molecular interaction of C. jejuni with eukaryotic cells is still poorly understood. Diverse behavior of the various C. jejuni phenotypes and/or the different strategies that C. jejuni exploits to adhere, invade, and survive in different cell types may explain, but also complicate, comparison and interpretation of reported results. Methodological issues may further blur scientific progress. A typical example is the gentamicin survival assay often used to estimate bacterial invasion into eukaryotic cells. Results obtained with this assay do not accurately measure the number of intracellular bacteria at a given time. Instead, they in fact reflect the outcome of a series of events including bacterial internalization, the resistance against the hostile intracellular milieu, and the adaptive capabilities of C. jejuni that are needed to survive the transition from the intracellular environment to growth on nutrient-rich agar plates in a different gas atmosphere. Recently, discovery of novel C. jejuni invasion and intracellular trafficking pathways was successful because these issues were taken into account (Watson and Galán 2008; van Alphen et al. 2008a). Despite these technicalities, several key steps in the C. jejuni infection of eukaryotic cells have been identified and are outlined below.
4.3 Adherence of C. jejuni to Mucosal Cells
Once close to the epithelial cells, C. jejuni can adhere to the cell surface through a number of adhesins (de Melo and Pechere 1990; Konkel et al. 1997; McSweegan and Walker 1986; Kervella et al. 1993). One identified adhesion is the 42-kDa protein JlpA. which mediates adherence to HEp-2 cells. This event can be inhibited with JlpA-specific antibodies or preincubation of the eukaryotic cells with purified JlpA protein (Jin et al. 2001). JlpA interacts with surface-exposed heat shock protein Hsp90α and is blocked by the Hsp90 inhibitor geldamycin (Jin et al. 2003). A JlpA-GST fusion protein triggers nuclear translocation of the transcription factor NF-kappaB and phosphorylation of p38 MAP kinase. This suggests that JlpA not only confers C. jejuni adherence but also elicits a proinflammatory response in the infected host cell (Jin et al. 2003).
A second protein with adhesive properties is CadF (Konkel et al. 1997). This protein, which may belong to the OmpA-like protein family, confers bacterial adhesion via binding of host cell fibronectin (Fn). The Fn binding domain of C. jejuni CadF has been identified as a single exposed amino acid domain of four residues (Konkel et al. 2005). Apart for CadF, Fn binding has also been proposed for C. jejuni flagellin, the major membrane protein MOMP, and LOS (Moser and Schroder 1997). The significance of this Fn binding for C. jejuni adherence is unknown.
Other putative C. jejuni adhesins are PEB1 (Pei et al. 1998) and certain LOS glycoforms (McSweegan and Walker 1986; Avril et al. 2006). A PEB1 mutant shows a 50- to 100-fold reduction in bacterial adherence to epithelial cells and a reduced colonization of mice (Pei et al. 1998). The protein binds to HeLa cell membranes (Kervella et al. 1993). More recently, PEB1 has been shown to be a conserved asparate/glutamate-binding protein that belongs to the family of cluster three binding proteins of bacterial ATP transporters (Leon-Kempis Mdel et al. 2006). Indeed, purified recombinant PEB1 binds L-aspartate and L-glutamate which may indicate that the protein is important in the utilization of in vivo carbon sources. Biochemical studies demonstrate that the majority of PEB1 protein resides in the periplasmic space and only a small portion is transported across the outer membrane (Leon-Kempis Mdel et al. 2006). The crystal structure of the protein further strengthened its role as periplasmic amino acid binding protein by demonstrating a ligand binding cleft, which could explain the high binding affinity for L-aspartate and L-glutamate (Muller et al. 2007). Whether the adherence-promoting properties of PEB1 relate to its importance in the uptake of amino acids necessary for bacterial growth is unknown. The function of C. jejuni LOS (McSweegan and Walker 1986) and capsule polysaccharide (Bachtiar et al. 2007) in bacterial adherence has not been systematically investigated, partly because their extensive intra- and interstrain structural heterogeneity.
At this time, the relative contribution of the various C. jejuni adhesins to the infection of mucosal cells is difficult to discern. In many studies, inactivation of each of the putative adhesins strongly reduces the association of C. jejuni with eukaryotic cells, indicating that they all are dominant adhesins. This suggests that either they act in a complex or display cell-type specificity. To our knowledge, tissue- or host-specific C. jejuni adhesins have thus far not been identified, but receptor identification for each of the adhesins may resolve this issue.
4.4 Mechanisms of C. jejuni Entry into Eukaryotic Cells
Analysis of intestinal biopsies of infected patients and primates (van Spreeuwel et al. 1985; Russell et al. 1993; Babakhani et al. 1993) as well as results from numerous in vitro studies (Newell and Pearson 1984; de Melo et al. 1989; Konkel et al. 1992a,b; Wassenaar et al. 1991; Babakhani et al. 1993; Oelschlaeger et al. 1993) indicate that C. jejuni is internalized by eukaryotic cells. Reported C. jejuni invasion levels, as mostly determined by the gentamycin assay, display huge variation between laboratories and are strongly dependent on multiplicity of infection used as an inoculum. The often-used C. jejuni strain 81–176 typically enters young semiconfluent INT-407 and Caco-2 cells in 2 h with an invasion efficiency of 1–2%, but efficiencies range from 0.001 to 4% (Hu and Kopecko 1999). In most studies, even with the most invasive strains, only one to three bacteria are internalized per cell (Biswas et al. 2000), much less than reported for other enteropathogens. However, when selected for the appropriate phenotype and dependent on the environmental conditions, uptake levels of 70–80% of the inoculum can be obtained within 2–4 h of infection (van Alphen et al. 2008a), suggesting that C. jejuni has the intrinsic ability to efficiently enter eukaryotic cells.
A key factor in virtually all C. jejuni uptake studies is the presence of functional flagella (Grant et al. 1993; Wassenaar et al. 1991; Yao et al. 1994; Szymanski et al. 1995; van Alphen et al. 2008a). C. jejuni that lack flagella, or carry a short flagellum consisting of only FlaB, show reduced invasion of epithelial cells (Wassenaar et al. 1991; Szymanski et al. 1995; Yao et al. 1994). Furthermore, C. jejuni with paralyzed flagella adhere but do not enter eukaryotic cells, suggesting that motility is a prerequisite for bacterial internalization (Yao et al. 1994). The hyperinvasiveness of CheY mutants, that display enhanced directional motility (Yao et al. 1997), also points in this direction.
The signals that drive the actual internalization process are still poorly defined. Upon contact with the cell surface, C. jejuni triggers membrane ruffling and invaginations, and is taken up with its polar tip first (Krause-Gruszczynska et al. 2007; Hu et al. 2008). The uptake process may require de novo protein synthesis (Oelschlaeger et al. 1993) and the flagellar secretion of the aforementioned FlaC, FspA, and Cia as the proteins. Inactivation of FlaC causes reduced invasion of C. jejuni into cultured epithelial cells but does not affect bacterial adherence (Song et al. 2004). The production and secretion of the Cia proteins is triggered by contact with host cells. However, the role of these proteins in the internalization event remains to be elucidated, as CiaB is obligatory for C. jejuni invasion in certain strains only (van Alphen et al. 2008a; Goon et al. 2006) and delivery of the secreted molecules to the host cells has not been demonstrated. Thus far, no function has been assigned to any of these proteins.
Recently, a novel highly efficient C. jejuni invasion mechanism has been identified that acts independently of CiaB or FlaC (van Alphen et al. 2008a) (Fig. 2). The highly invasive C. jejuni phenotype displays a remarkable route of invasion that yields on average 10–15 intracellular bacteria per epithelial cell. The C. jejuni first swims towards the subcellular space of cultured epithelial cells (a process termed “subvasion”) and then accesses the cell at the basal cell side. The subvasion process requires functional flagella. Molecular analysis of the selected highly subvasive bacteria indicated a change in the bacterial taxis system. This led to the discovery that C. jejuni subvasion can be directly controlled by the availability of nutrients (van Alphen and van Putten, unpublished results). The precise mechanism via which subcellular C. jejuni enters the eukaryotic cells, is still under investigation. One possible regulatory factor is the post-transcriptional regulator carbon starvation regulator CsrA. Inactivation of the protein, which is required for resistance of C. jejuni to oxidative stress, causes a strong increase in C. jejuni invasion despite reduced motility (Fields et al. 2008).
Other molecules uniquely involved in C. jejuni invasion are gamma-glutamyltranspeptidase (GGT) (Barnes et al. 2007), polysaccharide capsule (Karlyshev et al. 2000; Kanipes et al. 2004; Bacon et al. 2001), and sialylated LOS (Louwen et al. 2008). The underlying mechanisms, however, remain to be explored. The presence of the virulence plasmid pVir can enhance but is not required to trigger C. jejuni invasion (Bacon et al. 2002).
4.5 Cellular events Accompanying C. jejuni Internalization
Most studies are consistent with the scenario that C. jejuni-directed entry into epithelial cells proceeds via the local depolymerization of cortical actin filaments and the formation of microtubuli-based membrane projections (Krause-Gruszczynska et al. 2007; Watson and Galán 2008; Konkel et al. 1992a, b; Monteville et al. 2003; Oelschlaeger et al. 1993; Hu and Kopecko 1999). Microtubule depolymerizing agents, such as nocodazole, block C. jejuni invasion (Hu and Kopecko 1999; Oelschlaeger et al. 1993). The involvement of the actin cytoskeleton in C. jejuni uptake is not always found. Whether this relates to the presence of cortical cytochalasin D-insensitive actin filaments in certain cell types (Godman et al. 1980; Horvath and Kellie 1990) is unknown. Efficient C. jejuni internalization also requires caveolin-1 (Krause-Gruszczynska et al. 2007; Wooldridge et al. 1996; Hu et al. 2006a, b). However, as the entry process was shown to be dynamin-independent (Watson and Galán 2008), C. jejuni internalization is unlikely to occur via caveolea-mediated endocytosis. Instead, the caveolin-1 containing lipid membrane domains may be important for proper C. jejuni activation of tyrosine kinases (Watson and Galán 2008; Hu et al. 2006 b) and of the small Rho GTPases Rac 1 and Cdc42 (but not RhoA) that could drive the cytoskeletal rearrangements (Krause-Gruszczynska et al. 2007, Wooldridge et al. 1996; Hu et al. 2006a, b). Both the activation of the GTPases and C. jejuni uptake is blocked by the kinase inhibitors genistein, tyrphostin-46, wortmannin, and staurosporin. At least nine proteins become phosphorylated in C. jejuni-infected cells, including phosphoinositol 3-kinase and heterotrimeric G proteins (Wooldridge et al. 1996; Biswas et al. 2004). Their exact role in the uptake process, as well as the requirement of the release of Ca2+ from intracellular stores for C. jejuni entry (Hu et al. 2005), remain to be defined. Cell signaling studies with different mutants indicate that CadF and PEB1 are not essential for activation of the Rho GTPases (Krause-Gruszczynska et al. 2007). CadF does increase tyrosine phosphorylation of paxillin in focal adhesions and thus may contribute to cytoskeletal rearrangements during the Fn-mediated uptake at the basolateral cell surface (Monteville and Konkel 2002).
4.6 Intracellular Trafficking of C. jejuni
Once ingested by eukaryotic cells, C. jejuni resides in a membrane-bound cellular compartment and co-localizes with microtubules and the microtubule motor protein dynein during the entire invasion process (Hu and Kopecko 1999; Konkel et al. 1992b; Oelschlaeger et al. 1993; Russell and Blake 1994). In INT-407 cells, C. jejuni is able to replicate after an initial decline in the number of intracellular bacteria. Replication ultimately results in the deterioration of the epithelial monolayer. Bacterial iron acquisition is essential for intracellular survival in this cell line (Naikare et al. 2006). In HEp-2 epithelial cells, C. jejuni ultimately localize in vacuoles that show signs of phago-lysosome fusion and change from spiral to coccoid forms with a concomitant decrease in viability (de Melo et al. 1989).
A novel trafficking pathway was recently identified in semiconfluent T84 intestinal epithelial cells. In these cells, C. jejuni appears to traffick into a unique intracellular compartment and avoid delivery to lysosomes (Watson and Galán 2008) (Fig. 2). Once formed, the C. jejuni-containing vesicle is transported along microtubules to close proximity of the Golgi apparatus in the perinuclear region. Recovery of intracellular bacteria from this compartment requires oxygen-limiting conditions, a finding that could change conclusions from previously described invasion studies. The trafficking of C. jejuni to the non-lysosomal compartment is related to its entry mechanism as infection of Fc receptor-transfected cells with antibody-opsonized C. jejuni results in delivery of the bacteria to endosomes rather than the unique intracellular compartment (Watson and Galán 2008).
In polarized epithelial cells, C. jejuni is able to translocate to the basolateral surface to be released in the subcellular space (Fig. 2). During transit, C. jejuni remains with a membrane-bound compartment and there is only very limited replication intracellularly (Hu et al. 2008). The signals that drive this transcellular route are unknown.
4.7 Translocation of the Intestinal Mucosa
As well as through cellular invasion and the transcellular pathway, C. jejuni is also able to (transiently) disrupt tight junctions between polarized cells and thereby gain access to the subepithelial tissue via the paracellular route (Brás and Ketley 1999; Grant et al. 1993; Konkel et al. 1992b; MacCallum et al. 2005a) (Fig. 2). Infection of polarized T84 cells with C. jejuni decreases transepithelial electric resistance and causes a redistribution of the tight junction transmembrane protein occludin from an intercellular to an intracellular location, indicating alterations in the tight junctions (Chen et al. 2006). This event is enhanced in the presence of IFNγ (Rees et al. 2008). Once the tight junctions have been passed, C. jejuni can enter the eukaryotic cells at the basolateral surface as shown in EGTA-treated polarized cells (Monteville and Konkel 2002). The molecules that drive the transcellular migration and subsequent internalization process are largely undefined, although it can be imagined that the binding of Fn by CadF promotes basolateral uptake of C. jejuni through interaction with integrin receptors.
Several observations suggest that in vivo C. jejuni may exploit M cells to penetrate the intestinal barrier (Fig. 2). M cells are an important port of entry for a variety of pathogens including Salmonella (Siebers and Finlay 1996). In rabbit intestinal loop models, C. jejuni selectively associates with M cells (Everest et al. 1993; Walker et al. 1988). In differentiated polarized Caco-2 cells, the bacteria efficiently invade only approximately 5% of the cells that may represent M-like cells (Hu and Kopecko 1999; van Alphen and van Putten, unpublished results). The observed active penetration of the subcellular space by C. jejuni (van Alphen et al. 2008a) may reflect the spread of the pathogen from infected M cells into the subepithelial layer. These bacteria can subsequently invade the epithelial cells at cell basis. It has been speculated that, at low inocula (500–800 bacteria), M cells are primarily exploited by C. jejuni to traverse the epithelium, while at high inocula, the pathogen may invade villus epithelial cells, perhaps as a secondary event after loss of tight junction integrity (Everest 2005; Chen et al. 2006).
4.8 Interaction of C. jejuni with Professional Phagocytes
Whether C. jejuni can survive within professional phagocytes is still under debate. Both survival and replication inside monocytes and macrophages, but also induced CDT-dependent apoptosis, has been reported (Hickey et al. 2005; Kiehlbauch et al. 1985; Siegesmund et al. 2004). Clinical isolates of C. jejuni survive for several days in murine macrophages, whereby catalase plays an important role in providing resistance to hydrogen peroxide (Day et al. 2000). Others report that C. jejuni survival in human monocyte-derived macrophages is donor-dependent: in cells from most donors, C. jejuni is killed within 24–48 h inside the cells, while 10% of the donors carry monocytes that are unable to kill C. jejuni (Wassenaar et al. 1997). Intriguingly, C. jejuni DNA fragments can be detected in circulating human blood cells of distinct individuals for a period of up to 12 months, although no viable bacteria could be detected (van Rhijn et al. 2002). This may indicate the presence of either viable, non-culturable C. jejuni within these cells, or that the bacterium resides in a thus far unidentified niche within the body that serves as a reservoir for continuous C. jejuni infection of monocytes. In murine bone marrow-derived macrophages, C. jejuni is delivered to a lysosomal compartment and killed within 24 h of infection (Watson and Galán 2008). C. jejuni is also readily internalized by human dendritic cells and induces maturation and cytokine production in these cells (Hu et al. 2006a, b). In mice, this results in a Th1-effector response against C. jejuni (Rathinam et al. 2008).
5 Cellular Response to Infection
Despite its global relevance, the molecular basis of the C. jejuni intestinal pathology is still an enigma. One candidate bacterial factor contributing to the inflammatory pathology is CDT. The toxin not only induces cell cycle arrest but also activates the NF-kappaB inflammatory pathway (Whitehouse et al. 1998; Lara-Tejero and Galán 2001; Zheng et al. 2008). It can be imagined that the CDT-induced growth arrest affects the constant renewal of the epithelial cell lining and thereby disrupts the integrity of the intestinal barrier. This may allow bacterial tissue penetration and the induction of an inflammatory response (Whitehouse et al. 1998). Transcription profiling on Caco-2 cells infected with C. jejuni for 6 h indicated upregulation of genes involved in cell growth, gene transcription, steroid biosynthesis, and inflammation, but also in cell polarity, water movement, and solute transport (Rinella et al. 2006). The C. jejuni -specific altered gene expression was not observed in murine intestinal CT-62 cells, suggesting that the response may be species-specific. On the other hand, C. jejuni stimulates Na+ and Cl− secretion in infected rat ileum in a calcium-dependent and possibly protein kinase C-dependent fashion (Kanwar et al. 1995). Whether this effect is related to observed transcriptional alterations and/or the development of diarrhea awaits more knowledge of the bacterial factors that contribute to these responses
Analysis of the cellular signaling pathways indicates that C. jejuni induces a potent innate immune response that may contribute to the inflammatory pathology. C. jejuni infection of intestinal cells activates the transcription factors NF-kappaB and AP-1, causes phosphorylation of ERK and p38 mitogen-activated protein kinases and of JUN N-terminal protein kinase, and induces the basolateral secretion of proinflammatory mediators (Mellits et al. 2002; Jones et al. 2003; MacCallum et al. 2005b; Chen et al. 2006). Activation of the ERK and p38 mitogen-activated protein kinases requires de novo protein synthesis of C. jejuni factors that are produced upon contact with the host cells (Watson and Galán 2005). However, CDT (Zheng et al. 2008) and boiled extracts of C. jejuni also induce a proinflammatory transcriptional response in intestinal cells (Mellits et al. 2009). Other factors implicated in induction of the inflammatory response are C. jejuni lipoproteins and LOS, which activate the innate TLR2 and TLR4/MD2 signaling pathways, respectively (Zheng et al. 2008; Hu et al. 2006a, b). These effects appear particularly profound with damaged bacteria or isolated compounds (Hu et al. 2006a, b; de Zoete and van Putten, unpublished results). Maximal induction of the innate response with live C. jejuni appears to involve the intracellular Nod1, but not Nod2 innate immune receptor (Zilbauer et al. 2007). C. jejuni flagellin is a poor activator of TLR5 (Watson and Galán 2005; Andersen-Nissen et al. 2005; Johanesen and Dwinell 2006). Reconstitution of a recombinant C. jejuni flagellin that is able to activate TLR5, identified three small conserved regions in the flagellin backbone as the basis of the C. jejuni evasion of the TLR5 response (de Zoete and van Putten, unpublished results).
The activation of the innate immune response by C. jejuni results in the production of an array of proinflammatory cytokines and chemokines including IL-1α, IL-1β, IL-6, IL-6, and TNFα, but also of the neutrophil chemoattractant IL-8 (Hickey et al. 1999, 2000; Jones et al. 2003; Hu and Hickey 2005; Bakhiet et al. 2004; Johanesen and Dwinell 2006). These factors likely promote tissue damage and the recruitment of neutrophils and monocytes. The influx of these cell types at the site of infection is confirmed by immunohistochemistry on biopsies of infected patients. Produced innate effector molecules such as beta-defensins have potent antibacterial activity against C. jejuni (Zilbauer et al. 2005).
As chickens do not develop intestinal inflammation during C. jejuni colonization, a comparison of the avian and human innate immune system might shed light on molecular basis of inflammation. Analysis of the functional TLR repertoire of chickens indicates that this species has functional TLR4 and TLR5 receptors but lacks a TLR9 orthologue (Keestra and van Putten 2008; Keestra et al. 2008). The TLR2 complex differs from the mammalian species as it consists of a heterodimer of TLR2 and TLR16. TLR16 combines the specificity for di- and triacylpeptides of mammalian TLR1 and TLR6 in a single molecule (Keestra et al. 2007). Remarkably, the avian species appear to lack a functional MyD88-independent pathway needed for β-interferon production in response to LOS. This feature may explain the resistance of chickens to endotoxin shock (Keestra and van Putten 2008). Preliminary analysis of the response of individual chicken TLR receptors to C. jejuni provides, except for the different LOS response, no obvious basis for the lack of inflammation in C. jejuni infected chickens (de Zoete and van Putten, unpublished results).
6 Experimental Campylobacter Infection Models
Despite the identification of a number of putative virulence factors, their biological significance for C. jejuni pathology remains largely unknown. Understanding the pathogenesis of C. jejuni infections in vivo has long been hampered by the lack of suitable infection models. In its natural habitat of the avian species and other warm-blooded animals, C. jejuni colonizes the intestine but rarely causes disease (Beery et al. 1988). Thus, these species are suitable to investigate C. jejuni colonization rather than virulence. Similarly, murine infection models generally yield variable C. jejuni colonization but virtually no intestinal pathology unless the gut flora is limited or the immune system is compromised (Jesudason et al. 1989; Hodgson et al. 1998). In C3H SCID mice with limited gut flora, C. jejuni causes severe inflammation of the colon and cecum, but not diarrhea (Chang and Miller 2006). Intestinal colonization and pathology are observed only for motile, chemotaxis-proficient C. jejuni. NF-kappaB-deficient C57BL/29 mice display moderate intestinal inflammation, but only after infection with CDT-positive C. jejuni strains (Fox et al. 2004). Experimental infection of congenic C57BL/6 IL-10-deficient mice produces pathological lesions similar to those seen in humans, with C. jejuni present at paracellular junctions and at the basolateral surface of the epithelium (Mansfield et al. 2007). Mice deficient in the Toll-like receptor adaptor protein MyD88 can be persistently colonized by C. jejuni but do not develop pathology (Watson et al. 2007). In these mice, colonization requires N-glycosylation of proteins and capsule production (Watson et al. 2007), indicating these traits are required for colonization even when the innate immune response is attenuated.
The C. jejuni animal model closest resembling human infection is the oral infection of nonhuman primates. In experimentally infected macaques, C. jejuni causes an acute enterocolitis with bacteria invading the mucosa before the development of inflammation (Russell et al. 1989, 1993). Microscopy showed bacteria located in intestinal crypts, within surface epithelial cells and in the subepithelial tissue, indicative of mucosal penetration (Russell et al. 1989, 1993). These animals also display several clinical symptoms as observed during human infection, including bloody diarrhea. However, the nonhuman primate animal model is rarely used, mainly on ethical grounds. A promising alternative model involves the use of gnotobiotic piglets. Infection of colostrum-deprived newborn piglets with C. jejuni results in clinical symptoms and histopathology similar to those observed in humans infected with C. jejuni. The bacteria cause gross lesions in the large intestine with edema, neutrophil infiltration, and sloughing of epithelial cells, and hemorrhage or increased mucus production. At the cellular level, bacteria are found inside intestinal epithelial cells as well as in the underlying tissue (Babakhani et al. 1993; Boosinger and Powe 1988).
Overall, the in vivo infection models suggest that in most animals the indigenous gut flora and/or a well-developed host immune system may limit C. jejuni intestinal pathology. The use of animals with limited gut flora and/or immune deficiencies as C. jejuni infection models may shed light on the molecular basis and species-specificity of the C. jejuni-induced pathology, and provide information on the bacterial phenotype and metabolic status of the bacteria compared with those isolated from the human intestine.
7 Conclusions and Outlook
A primary challenge in C. jejuni research remains the discovery of the molecular basis of the pathogenic behavior of C. jejuni in humans compared to the commensal behavior in most other species, in particular poultry. Dissection of the virulence strategies of C. jejuni is seriously hampered by the huge phenotype diversity. Extensive surface variation and the broad adaptation potential appear to provide the pathogen with an array of seemingly redundant tools to exploit host cell biology. Strain differences further complicate functional analysis of C. jejuni pathogenicity. Yet, current knowledge indicates flagella-mediated motility, chemotaxis, and penetration of mucus as key determinants of bacterial colonization in all species. The induction of pathology appears to require damage of the integrity of the epithelium lining the mucosa, either via toxin production, invasion of epithelial cells, weakening of cellular tight junctions, and/or traversal of intestinal M cells. Bacterial products as well as cellular damage may elicit an inflammatory response that contributes to the development of pathology. One key determinant of the course of an infection may be the local microenvironment at the site of infection. C. jejuni appears to be able to quickly adapt its phenotype to changing microenvironments like the availability of oxygen and nutrient sources. Recent progress in the understanding of the C. jejuni genome diversity, gene regulation, and its dynamic behavior in different environments has paved the way to finally understand and attack the virulence strategies of this important pathogen.
References
Allen KJ, Griffiths MW (2001) Effect of environmental and chemotactic stimuli on the activity of the Campylobacter jejuni flaA sigma(28) promoter. FEMS Microbiol Lett 205:43–48
Allos BM, Blaser MJ (1995) Campylobacter jejuni and the expanding spectrum of related infections. Clin Infect Dis 20:1092–1099
Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, Aderem A (2005) Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci USA 102:9247–9252
Ashgar SS, Oldfield NJ, Wooldridge KG, Jones MA, Irving GJ, Turner DP, Ala'Aldeen DA (2007) CapA, an autotransporter protein of Campylobacter jejuni, mediates association with human epithelial cells and colonization of the chicken gut. J Bacteriol 189:1856–1865
Avril T, Wagner ER, Willison HJ, Crocker PR (2006) Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect Immun 74:4133–4141
Babakhani FK, Bradley GA, Joens LA (1993) Newborn piglet model for campylobacteriosis. Infect Immun 61:3466–3475
Bacon DJ, Alm RA, Hu L, Hickey TE, Ewing CP, Batchelor RA, Trust TJ, Guerry P (2002) DNA sequence and mutational analyses of the pVir plasmid of Campylobacter jejuni 81–176. Infect Immun 70:6242–6250
Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P (2001) A phase-variable capsule is involved in virulence of Campylobacter jejuni 81–176. Mol Microbiol 40:769–777
Bakhiet M, Al-Salloom FS, Qareiballa A, Bindayna K, Farid I, Botta GA (2004) Induction of alpha and beta chemokines by intestinal epithelial cells stimulated with Campylobacter jejuni. J Infect 48:236–244
Barnes IH, Bagnall MC, Browning DD, Thompson SA, Manning G, Newell DG (2007) Gamma-glutamyl transpeptidase has a role in the persistent colonization of the avian gut by Campylobacter jejuni. Microb Pathog 43:198–207
Beery JT, Hugdahl MB, Doyle MP (1988) Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl Environ Microbiol 54:2365–2370
Biswas D, Itoh K, Sasakawa C (2000) Uptake pathways of clinical and healthy animal isolates of Campylobacter jejuni into INT-407 cells. FEMS Immunol Med Microbiol 29:203–211
Biswas D, Niwa H, Itoh K (2004) Infection with Campylobacter jejuni induces tyrosine-phosphorylated proteins into INT-407 cells. Microbiol Immunol 48:221–228
Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ (1988) Experimental Campylobacter jejuni infection in humans. J Infect Dis 157:472–479
Boosinger TR, Powe TA (1988) Campylobacter jejuni infections in gnotobiotic pigs. Am J Vet Res 49:456–458
Brás AM, Ketley JM (1999) Transcellular translocation of Campylobacter jejuni across human polarised epithelial monolayers. FEMS Microbiol Lett 179:209–215
Burnens A, Stucki U, Nicolet J, Frey J (1995) Identification and characterization of an immunogenic outer membrane protein of Campylobacter jejuni. J Clin Microbiol 33:2826–2832
Buzby JC, Roberts T (1997) Economic costs and trade impacts of microbial foodborne illness. World Health Stat Q 50:57–66
Chang C, Miller JF (2006) Campylobacter jejuni colonization of mice with limited enteric flora. Infect Immun 74:5261–5271
Chen ML, Ge Z, Fox JG, Schauer DB (2006) Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni. Infect Immun 74:6581–6589
Clark CG, Beeston A, Bryden L, Wang G, Barton C, Cuff W, Gilmour MW, Ng LK (2007) Phylogenetic relationships of Campylobacter jejuni based on porA sequences. Can J Microbiol 53:27–38
Clark CG, Ng LK (2008) Sequence variability of Campylobacter temperate bacteriophages. BMC Microbiol 8:49
Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Obi CL (2002) Human campylobacteriosis in developing countries. Emerg Infect Dis 8:237–244
Colgan T, Lambert JR, Newman A, Luk SC (1980) Campylobacter jejuni enterocolitis. A clinicopathologic study. Arch Pathol Lab Med 104:571–574
Corcoran AT, Annuk H, Moran AP (2006) The structure of the lipid anchor of Campylobacter jejuni polysaccharide. FEMS Microbiol Lett 257:228–235
Coward C, Grant AJ, Swift C, Philp J, Towler R, Heydarian M, Frost JA, Maskell DJ (2006) Phase-variable surface structures are required for infection of Campylobacter jejuni by bacteriophages. Appl Environ Microbiol 72:4638–4647
Day WA Jr, Sajecki JL, Pitts TM, Joens LA (2000) Role of catalase in Campylobacter jejuni intracellular survival. Infect Immun 68:6337–6345
Dé E, Jullien M, Labesse G, Pagès JM, Molle G, Bolla JM (2000) MOMP (major outer membrane protein) of Campylobacter jejuni; a versatile pore-forming protein. FEBS Lett 469:93–97
De Melo MA, Gabbiani G, Pechere JC (1989) Cellular events and intracellular survival of Campylobacter jejuni during infection of HEp-2 cells. Infect Immun 57:2214–2222
de Melo MA, Pechere JC (1990) Identification of Campylobacter jejuni surface proteins that bind to eucaryotic cells in vitro. Infect Immun 58:1749–1756
Dzieciatkowska M, Brochu D, van Belkum A, Heikema AP, Yuki N, Houliston RS, Richards JC, Gilbert M, Li J (2007) Mass spectrometric analysis of intact lipooligosaccharide: direct evidence for O-acetylated sialic acids and discovery of O-linked glycine expressed by Campylobacter jejuni. Biochemistry 46:14704–14714
Elliott KT, Zhulin IB, Stuckey JA, DiRita VJ (2009) Conserved residues in the HAMP domain define a new family of proposed bipartite energy taxis receptors. J Bacteriol 191:375–387
Elliott KT, DiRita VJ (2008) Characterization of CetA and CetB, a bipartite energy taxis system in Campylobacter jejuni. Mol Microbiol 69:1091–103
Everest PH, Cole AT, Hawkey CJ, Knutton S, Goossens H, Butzler JP, Ketley JM, Williams PH (1993) Roles of leukotriene B4, prostaglandin E2, and cyclic AMP in Campylobacter jejuni-induced intestinal fluid secretion. Infect Immun 61:4885–4887
Everest P (2005) Campylobacter spp. and the ability to elicit intestinal inflammatory responses. In: Ketley JM, Konkel ME (eds) Campylobacter: molecular and cellular biology. Horizon Bioscience, Norfolk, pp 421–434
Ferrero RL, Lee A (1988) Motility of Campylobacter jejuni in a viscous environment: comparison with conventional rod-shaped bacteria. J Gen Microbiol 134:53–59
Fields JA, Thompson SA (2008) Campylobacter jejuni CsrA mediates oxidative stress responses, biofilm formation, and host cell invasion. J Bacteriol 190:3411–3416
Fouts DE, Mongodin EF, Mandrell RE, Miller WG, Rasko DA, Ravel J, Brinkac LM, DeBoy RT, Parker CT, Daugherty SC, Dodson RJ, Durkin AS, Madupu R, Sullivan SA, Shetty JU, Ayodeji MA, Shvartsbeyn A, Schatz MC, Badger JH, Fraser CM, Nelson KE (2005) Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol 3:e15
Fox JG, Rogers AB, Whary MT, Ge Z, Taylor NS, Xu S, Horwitz BH, Erdman SE (2004) Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect Immun 72:1116–1125
Fry BN, Feng S, Chen YY, Newell DG, Coloe PJ, Korolik V (2000) The galE gene of Campylobacter jejuni is involved in lipopolysaccharide synthesis and virulence. Infect Immun 68:2594–2601
Gaasbeek EJ, Wagenaar JA, Guilhabert MR, Wösten MM, van Putten JP, van der Graaf-van Bloois L, Parker CT, van der Wal FJ (2009) A DNase encoded by integrated element CJIE1 inhibits natural transformation of Campylobacter jejuni. J Bacteriol. doi:10.1128/JB.01430-08
Galkin VE, Yu X, Bielnicki J, Heuser J, Ewing CP, Guerry P, Egelman EH (2008) Divergence of quaternary structures among bacterial flagellar filaments. Science 320:382–385
Gilbert M, Karwaski MF, Bernatchez S, Young NM, Taboada E, Michniewicz J, Cunningham AM, Wakarchuk WW (2002) The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen. Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J Biol Chem 277:327–337
Godman G, Woda B, Kolberg R, Berl S (1980) Redistribution of contractile and cytoskeletal components induced by cytochalasin. II. In HeLa and HEp2 cells. Eur J Cell Biol 22:745–754
Godschalk PC, Kuijf ML, Li J, St Michael F, Ang CW, Jacobs BC, Karwaski MF, Brochu D, Moterassed A, Endtz HP, van Belkum A, Gilbert M (2007) Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barre and Miller Fisher syndromes. Infect Immun 75:1245–1254
Golden NJ, Acheson DW (2002) Identification of motility and autoagglutination Campylobacter jejuni mutants by random transposon mutagenesis. Infect Immun 70:1761–1771
Goon S, Kelly JF, Logan SM, Ewing CP, Guerry P (2003) Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the Cj1293 gene. Mol Microbiol 50:659–671
Goon S, Ewing CP, Lorenzo M, Pattarini D, Majam G, Guerry P (2006) A sigma28-regulated nonflagella gene contributes to virulence of Campylobacter jejuni 81–176. Infect Immun 74:769–772
Grant CC, Konkel ME, Cieplak W Jr, Tompkins LS (1993) Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun 61:1764–1771
Guccione E, Leon-Kempis Mdel R, Pearson BM, Hitchin E, Mulholland F, van Diemen PM, Stevens MP, Kelly DJ (2008) Amino acid-dependent growth of Campylobacter jejuni: key roles for aspartase (AspA) under microaerobic and oxygen-limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol Microbiol 69:77–93
Guerry P (2007) Campylobacter flagella: not just for motility. Trends Microbiol 15:456–461
Guerry P, Ewing CP, Schirm M, Lorenzo M, Kelly J, Pattarini D, Majam G, Thibault P, Logan S (2006) Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol Microbiol 60:299–311
Guerry P, Szymanski CM (2008) Campylobacter sugars sticking out. Trends Microbiol 16:428–435
Guerry P, Szymanski CM, Prendergast MM, Hickey TE, Ewing CP, Pattarini DL, Moran AP (2002) Phase variation of Campylobacter jejuni 81–176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect Immun 70:787–793
Havelaar AH, van Pelt W, Ang CW, Wagenaar JA, van Putten JPM, Gross U, Newell DG (2009) Immunity to Campylobacter: its role in risk assessment and epidemiology. Crit Rev Microbiol. 35:1–22
He Y, Frye JG, Strobaugh TP, Chen CY (2008) Analysis of AI-2/LuxS-dependent transcription in Campylobacter jejuni strain 81–176. Foodborne Pathog Dis 5:399–415
Hendrixson DR, Akerley BJ, DiRita VJ (2001) Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol Microbiol 40:214–224
Hendrixson DR, DiRita VJ (2002) Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol Microbiol 52:471–484
Hendrixson DR, DiRita VJ (2003) Transcription of sigma54-dependent but not sigma28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol Microbiol 50:687–702
Hendrixson DR, DiRita VJ (2004) Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol Microbiol 52:471–484
Hickey TE, Baqar S, Bourgeois AL, Ewing CP, Guerry P (1999) Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect Immun 67:88–93
Hickey TE, Majam G, Guerry P (2005) Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infect Immun 73:5194–5197
Hickey TE, McVeigh AL, Scott DA, Michielutti RE, Bixby A, Carroll SA, Bourgeois AL, Guerry P (2000) Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect Immun 68:6535–6541
Hodgson AE, McBride BW, Hudson MJ, Hall G, Leach SA (1998) Experimental campylobacter infection and diarrhoea in immunodeficient mice. J Med Microbiol 47:799–809
Hofreuter D, Novik V, Galán JE (2008) Metabolic diversity in Campylobacter jejuni enhances specific tissue colonization. Cell Host Microbe 4:425–433
Hofreuter D, Tsai J, Watson RO, Novik V, Altman B, Benitez M, Clark C, Perbost C, Jarvie T, Du L, Galán JE (2006) Unique features of a highly pathogenic Campylobacter jejuni strain. Infect Immun 74:4694–4707
Horvath AR, Kellie S (1990) Regulation of integrin mobility and cytoskeletal association in normal and RSV-transformed chick embryo fibroblasts. J Cell Sci 97:307–315
Hu L, Bray MD, Osorio M, Kopecko DJ (2006a) Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect Immun 74:2697–2705
Hu L, Hickey TE (2005) Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect Immun 73:4437–4440
Hu L, Kopecko DJ (1999) Campylobacter jejuni 81–176 associates with microtubules and dynein during invasion of human intestinal cells. Infect Immun 67:4171–4182
Hu L, McDaniel JP, Kopecko DJ (2006b) Signal transduction events involved in human epithelial cell invasion by Campylobacter jejuni 81–176. Microb Pathog 40:91–100
Hu L, Raybourne RB, Kopecko DJ (2005) Ca2+ release from host intracellular stores and related signal transduction during Campylobacter jejuni 81–176 internalization into human intestinal cells. Microbiology 151:3097–3105
Hu L, Tall BD, Curtis SK, Kopecko DJ (2008) Enhanced microscopic definition of Campylobacter jejuni 81–176 adherence to, invasion of, translocation across, and exocytosis from polarized human intestinal Caco-2 cells. Infect Immun 76:5294–5304
Hugdahl MB, Beery JT, Doyle MP (1988) Chemotactic behavior of Campylobacter jejuni. Infect Immun 56:1560–1566
Jesudason MV, Hentges DJ, Pongpech P (1989) Colonization of mice by Campylobacter jejuni. Infect Immun 57:2279–2282
Jin S, Joe A, Lynett J, Hani EK, Sherman P, Chan VL (2001) JlpA, a novel surface-exposed lipoprotein specific to Campylobacter jejuni, mediates adherence to host epithelial cells. Mol Microbiol 39:1225–1236
Jin S, Song YC, Emili A, Sherman PM, Chan VL (2003) JlpA of Campylobacter jejuni interacts with surface-exposed heat shock protein 90alpha and triggers signalling pathways leading to the activation of NF-kappaB and p38 MAP kinase in epithelial cells. Cell Microbiol 5:165–174
Johanesen PA, Dwinell MB (2006) Flagellin-independent regulation of chemokine host defense in Campylobacter jejuni-infected intestinal epithelium. Infect Immun 74:3437–3447
Jones MA, Totemeyer S, Maskell DJ, Bryant CE, Barrow PA (2003) Induction of proinflammatory responses in the human monocytic cell line THP-1 by Campylobacter jejuni. Infect Immun 71:2626–2633
Joshua GW, Guthrie-Irons C, Karlyshev AV, Wren BW (2006) Biofilm formation in Campylobacter jejuni. Microbiology 152:387–396
Kalmokoff M, Lanthier P, Tremblay TL, Foss M, Lau PC, Sanders G, Austin J, Kelly J, Szymanski CM (2006) Proteomic analysis of Campylobacter jejuni 11168 biofilms reveals a role for the motility complex in biofilm formation. J Bacteriol 188:4312–4320
Kanipes MI, Holder LC, Corcoran AT, Moran AP, Guerry P (2004) A deep-rough mutant of Campylobacter jejuni 81–176 is noninvasive for intestinal epithelial cells. Infect Immun 72:2452–2455
Kanwar RK, Ganguly NK, Kumar L, Rakesh J, Panigrahi D, Walia BN (1995) Calcium and protein kinase C play an important role in Campylobacter jejuni-induced changes in Na+ and Cl- transport in rat ileum in vitro. Biochim Biophys Acta 1270:179–192
Karlyshev AV, Champion OL, Churcher C, Brisson JR, Jarrell HC, Gilbert M, Brochu D, St Michael F, Li J, Wakarchuk WW, Goodhead I, Sanders M, Stevens K, White B, Parkhill J, Wren BW, Szymanski CM (2005) Analysis of Campylobacter jejuni capsular loci reveals multiple mechanisms for the generation of structural diversity and the ability to form complex heptoses. Mol Microbiol 55:90–103
Karlyshev AV, Everest P, Linton D, Cawthraw S, Newell DG, Wren BW (2004) The Campylobacter jejuni general glycosylation system is important for attachment to human epithelial cells and in the colonization of chicks. Microbiology 150:1957–1964
Karlyshev AV, Linton D, Gregson NA, Lastovica AJ, Wren BW (2000) Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol Microbiol 35:529–541
Karlyshev AV, Linton D, Gregson NA, Wren BW (2002) A novel paralogous gene family involved in phase-variable flagella-mediated motility in Campylobacter jejuni. Microbiology 148:473–480
Karlyshev AV, McCrossan MV, Wren BW (2001) Demonstration of polysaccharide capsule in Campylobacter jejuni using electron microscopy. Infect Immun 69:5921–24
Keestra AM, van Putten JPM (2008) Unique properties of the chicken TLR4/MD-2 complex: selective lipopolysaccharide activation of the MyD88-dependent pathway. J Immunol 181:4354–4362
Keestra AM, de Zoete MR, van Aubel RA, van Putten JPM (2008) Functional characterization of chicken TLR5 reveals species-specific recognition of flagellin. Mol Immunol 45:1298–1307
Keestra AM, de Zoete MR, van Aubel RA, van Putten JPM (2007) The central leucine-rich repeat region of chicken TLR16 dictates unique ligand specificity and species-specific interaction with TLR2. J Immunol 178:7110–7119
Kervella M, Pages JM, Pei Z, Grollier G, Blaser MJ, Fauchere JL (1993) Isolation and characterization of two Campylobacter glycine-extracted proteins that bind to HeLa cell membranes. Infect Immun 61:3440–3448
Kiehlbauch JA, Albach RA, Baum LL, Chang KP (1985) Phagocytosis of Campylobacter jejuni and its intracellular survival in mononuclear phagocytes. Infect Immun 48:446–451
Konkel ME, Christensen JE, Keech AM, Monteville MR, Klena JD, Garvis SG (2005) Identification of a fibronectin-binding domain within the Campylobacter jejuni CadF protein. Mol Microbiol 57:1022–1035
Konkel ME, Garvis SG, Tipton SL, Anderson DE Jr, Cieplak W Jr (1997) Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol Microbiol 24:953–963
Konkel ME, Hayes SF, Joens LA, Cieplak W Jr (1992a) Characteristics of the internalization and intracellular survival of Campylobacter jejuni in human epithelial cell cultures. Microb Pathog 13:357–370
Konkel ME, Kim BJ, Rivera-Amill V, Garvis SG (1999) Identification of proteins required for the internalization of Campylobacter jejuni into cultured mammalian cells. Adv Exp Med Biol 473:215–224
Konkel ME, Mead DJ, Cieplak W Jr (1996) Cloning, sequencing, and expression of a gene from Campylobacter jejuni encoding a protein (Omp18) with similarity to peptidoglycan-associated lipoproteins. Infect Immun 64:1850–1853
Konkel ME, Mead DJ, Hayes SF, Cieplak W Jr (1992b) Translocation of Campylobacter jejuni across human polarized epithelial cell monolayer cultures. J Infect Dis 166:308–315
Kowarik M, Young NM, Numao S, Schulz BL, Hug I, Callewaert N, Mills DC, Watson DC, Hernandez M, Kelly JF, Wacker M, Aebi M (2006) Definition of the bacterial N-glycosylation site consensus sequence. EMBO J 25:1957–1966
Krause-Gruszczynska M, Rohde M, Hartig R, Genth H, Schmidt G, Keo T, Konig W, Miller WG, Konkel ME, Backert S (2007) Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni. Cell Microbiol 9:2431–2444
Lara-Tejero M, Galán JE (2001) CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect Immun 69:4358–4365
Lecuit M, Abachin E, Martin A, Poyart C, Pochart P, Suarez F, Bengoufa D, Feuillard J, Lavergne A, Gordon JI, Berche P, Guillevin L, Lortholary O (2004) Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med 350:239–248
Lee A, O'Rourke JL, Barrington PJ, Trust TJ (1986) Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni : a mouse cecal model. Infect Immun 51:536–546
Lee RB, Hassane DC, Cottle DL, Pickett CL (2003) Interactions of Campylobacter jejuni cytolethal distending toxin subunits CdtA and CdtC with HeLa cells. Infect Immun 71:4883–4890
Leon-Kempis Mdel R, Guccione E, Mulholland F, Williamson MP, Kelly DJ (2006) The Campylobacter jejuni PEB1a adhesin is an aspartate/glutamate-binding protein of an ABC transporter essential for microaerobic growth on dicarboxylic amino acids. Mol Microbiol 60:1262–1275
Linton D, Gilbert M, Hitchen PG, Dell A, Morris HR, Wakarchuk WW, Gregson NA, Wren BW (2000) Phase variation of a beta-1, 3 galactosyltransferase involved in generation of the ganglioside GM1-like lipo-oligosaccharide of Campylobacter jejuni. Mol Microbiol 37:501–514
Logan SM, Hui JP, Vinogradov E, Aubry AJ, Melanson JE, Kelly JF, Nothaft H, Soo EC (2009) Identification of novel carbohydrate modifications on Campylobacter jejuni 11168 flagellin using metabolomics-based approaches. FEBS J 276:1014–1023
Logan SM, Kelly JF, Thibault P, Ewing CP, Guerry P (2002) Structural heterogeneity of carbohydrate modifications affects serospecificity of Campylobacter flagellins. Mol Microbiol 46:587–597
Louwen R, Heikema A, van Belkum A, Ott A, Gilbert M, Ang W, Endtz HP, Bergman MP, Nieuwenhuis EE (2008) The sialylated lipooligosaccharide outer core in Campylobacter jejuni is an important determinant for epithelial cell invasion. Infect Immun 76:4431–4438
Louwen RP, van Belkum A, Wagenaar JA, Doorduyn Y, Achterberg R, Endtz HP (2006) Lack of association between the presence of the pVir plasmid and bloody diarrhea in Campylobacter jejuni enteritis. J Clin Microbiol 44:1867–1868
MacCallum A, Haddock G, Everest PH (2005a) Campylobacter jejuni activates mitogen-activated protein kinases in Caco-2 cell monolayers and in vitro infected primary human colonic tissue. Microbiology 151:2765–2772
MacCallum A, Hardy SP, Everest PH (2005b) Campylobacter jejuni inhibits the absorptive transport functions of Caco-2 cells and disrupts cellular tight junctions. Microbiology 151:2451–2458
MacKichan JK, Gaynor EC, Chang C, Cawthraw S, Newell DG, Miller JF, Falkow S (2004) The Campylobacter jejuni dccRS two-component system is required for optimal in vivo colonization but is dispensable for in vitro growth. Mol Microbiol 54:1269–1286
Malik-Kale P, Parker CT, Konkel ME (2008) Culture of Campylobacter jejuni with sodium deoxycholate induces virulence gene expression. J Bacteriol 190:2286–2297
Mansfield LS, Bell JA, Wilson DL, Murphy AJ, Elsheikha HM, Rathinam VA, Fierro BR, Linz JE, Young VB (2007) C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect Immun 75:1099–1115
Marchant J, Wren B, Ketley J (2002) Exploiting genome sequence: predictions for mechanisms of Campylobacter chemotaxis. Trends Microbiol 10:155–159
McLennan MK, Ringoir DD, Frirdich E, Svensson SL, Wells DH, Jarrell H, Szymanski CM, Gaynor EC (2008) Campylobacter jejuni biofilms up-regulated in the absence of the stringent response utilize a calcofluor white-reactive polysaccharide. J Bacteriol 190:1097–1107
McNally DJ, Hui JP, Aubry AJ, Mui KK, Guerry P, Brisson JR, Logan SM, Soo EC (2006) Functional characterization of the flagellar glycosylation locus in Campylobacter jejuni 81–176 using a focused metabolomics approach. J Biol Chem 281:18489–18498
McNally DJ, Lamoureux MP, Karlyshev AV, Fiori LM, Li J, Thacker G, Coleman RA, Khieu NH, Wren BW, Brisson JR, Jarrell HC, Szymanski CM (2007) Commonality and biosynthesis of the O-methyl phosphoramidate capsule modification in Campylobacter jejuni. J Biol Chem 282:28566–28576
McSweegan E, Burr DH, Walker RI (1987) Intestinal mucus gel and secretory antibody are barriers to Campylobacter jejuni adherence to INT 407 cells. Infect Immun 55:1431–1435
McSweegan E, Walker RI (1986) Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun 53:141–148
Meinersmann RJ, Rigsby WE, Stern NJ, Kelley LC, Hill JE, Doyle MP (1991) Comparative study of colonizing and noncolonizing Campylobacter jejuni. Am J Vet Res 52:1518–1522
Mellits KH, Mullen J, Wand M, Armbruster G, Patel A, Connerton PL, Skelly M, Connerton IF (2002) Activation of the transcription factor NF-kappaB by Campylobacter jejuni. Microbiology 148:2753–2763
Mellits KH, Connerton IF, Loughlin MF, Clarke P, Smith J, Dillon E, Connerton PL, Mulholland F, Hawkey CJ (2009) Induction of a chemoattractant transcriptional response by a Campylobacter jejuni boiled cell extract in colonocytes. BMC Microbiol. 9:28
Medema GJ, Teunis PF, Havelaar AH, Haas CN (1996) Assessment of the dose-response relationship of Campylobacter jejuni. Int J Food Microbiol 30:101–111
Miller WG, Heath S, Mandrell RE (2007) Cryptic plasmids isolated from Campylobacter strains represent multiple, novel incompatibility groups. Plasmid 57:108–117
Monteville MR, Konkel ME (2002) Fibronectin-facilitated invasion of T84 eukaryotic cells by Campylobacter jejuni occurs preferentially at the basolateral cell surface. Infect Immun 70:6665–6671
Monteville MR, Yoon JE, Konkel ME (2003) Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni requires the CadF outer-membrane protein and microfilament reorganization. Microbiology 149:153–165
Moran AP (1995) Biological and serological characterization of Campylobacter jejuni lipopolysaccharides with deviating core and lipid A structures. FEMS Immunol Med Microbiol 11:121–130
Moran AP (1997) Structure and conserved characteristics of Campylobacter jejuni lipopolysaccharides. J Infect Dis 176(Suppl 2):S115–121
Morooka T, Umeda A, Amako K (1985) Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol 131:1973–1980
Moser I, Schröder W (1997) Hydrophobic characterization of thermophilic Campylobacter species and adhesion to INT 407 cell membranes and fibronectin. Microb Pathog 22:155–164
Moser I, Schroeder W, Salnikow J (1997) Campylobacter jejuni major outer membrane protein and a 59-kDa protein are involved in binding to fibronectin and INT 407 cell membranes. FEMS Microbiol Lett 157:233–238
Muller A, Leon-Kempis MD, Dodson E, Wilson KS, Wilkinson AJ, Kelly DJ (2007) A bacterial virulence factor with a dual role as an adhesin and a solute-binding protein: The crystal Structure at 1.5 A resolution of the PEB1a protein from the food-borne human pathogen Campylobacter jejuni. J Mol Biol 372:160–171
Naikare H, Palyada K, Panciera R, Marlow D, Stintzi A (2006) Major role for FeoB in Campylobacter jejuni ferrous iron acquisition, gut colonization, and intracellular survival. Infect Immun 74:5433–5444
Newell DG, Pearson A (1984) The invasion of epithelial cell lines and the intestinal epithelium of infant mice by Campylobacter jejuni/coli. J Diarrhoeal Dis Res 2:19–26
Oelschlaeger TA, Guerry P, Kopecko DJ (1993) Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA 90:6884–6888
Palyada K, Threadgill D, Stintzi A (2004) Iron acquisition and regulation in Campylobacter jejuni. J Bacteriol 186:4714–4729
Parker CT, Gilbert M, Yuki N, Endtz HP, Mandrell RE (2008) Characterization of lipooligosaccharide-biosynthetic loci of Campylobacter jejuni reveals new lipooligosaccharide classes: evidence of mosaic organizations. J Bacteriol 190:5681–5689
Parker CT, Quinones B, Miller WG, Horn ST, Mandrell RE (2006) Comparative genomic analysis of Campylobacter jejuni strains reveals diversity due to genomic elements similar to those present in C. jejuni strain RM1221. J Clin Microbiol 44:4125–4135
Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG (2000) The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668
Pearson BM, Pin C, Wright J, I'Anson K, Humphrey T, Wells JM (2003) Comparative genome analysis of Campylobacter jejuni using whole genome DNA microarrays. FEBS Lett 554:224–230
Pei Z, Burucoa C, Grignon B, Baqar S, Huang XZ, Kopecko DJ, Bourgeois AL, Fauchere JL, Blaser MJ (1998) Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect Immun 66:938–943
Poly F, Ewing C, Goon S, Hickey TE, Rockabrand D, Majam G, Lee L, Phan J, Savarino NJ, Guerry P (2007) Heterogeneity of a Campylobacter jejuni protein that is secreted through the flagellar filament. Infect Immun 75:3859–3867
Poly F, Threadgill D, Stintzi A (2005) Genomic diversity in Campylobacter jejuni: identification of C. jejuni 81–176-specific genes. J Clin Microbiol 43:2330–2338
Quinones B, Miller WG, Bates AH, Mandrell RE (2009) Autoinducer-2 production in Campylobacter jejuni contributes to chicken colonization. Appl Environ Microbiol 75:281–285
Rathinam VA, Hoag KA, Mansfield LS (2008) Dendritic cells from C57BL/6 mice undergo activation and induce Th1-effector cell responses against Campylobacter jejuni. Microbes Infect 10:1316–1324
Rees LE, Cogan TA, Dodson AL, Birchall MA, Bailey M, Humphrey TJ (2008) Campylobacter and IFNgamma interact to cause a rapid loss of epithelial barrier integrity. Inflamm Bowel Dis 14:303–309
Rinella ES, Eversley CD, Carroll IM, Andrus JM, Threadgill DW, Threadgill DS (2006) Human epithelial-specific response to pathogenic Campylobacter jejuni. FEMS Microbiol Lett 262:236–243
Rivera-Amill V, Kim BJ, Seshu J, Konkel ME (2001) Secretion of the virulence-associated Campylobacter invasion antigens from Campylobacter jejunirequires a stimulatory signal. J Infect Dis 183:1607–1616
Russell RG, Blake DC Jr (1994) Cell association and invasion of Caco-2 cells by Campylobacter jejuni. Infect Immun 62:3773–3779
Russell RG, Blaser MJ, Sarmiento JI, Fox J (1989) Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect Immun 57:1438–1444
Russell RG, O'Donnoghue M, Blake DC Jr, Zulty J, DeTolla LJ (1993) Early colonic damage and invasion of Campylobacter jejuni in experimentally challenged infant Macaca mulatta. J Infect Dis 168:210–215
Sellars MJ, Hall SJ, Kelly DJ (2002) Growth of Campylobacter jejunisupported by respiration of fumarate, nitrate, nitrite, trimethylamine-N-oxide, or dimethyl sulfoxide requires oxygen. J Bacteriol 184:4187–4196
Shigematsu M, Umeda A, Fujimoto S, Amako K (1998) Spirochaete-like swimming mode of Campylobacter jejuni in a viscous environment. J Med Microbiol 47:521–526
Siebers A, Finlay BB (1996) M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol 4:22–29
Siegesmund AM, Konkel ME, Klena JD, Mixter PF (2004) Campylobacter jejuni infection of differentiated THP-1 macrophages results in interleukin 1 beta release and caspase-1-independent apoptosis. Microbiology 150:561–569
Smith JL, Bayles DO (2006) The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit Rev Microbiol 32:227–248
Song YC, Jin S, Louie H, Ng D, Lau R, Zhang Y, Weerasekera R, Al Rashid S, Ward LA, Der SD, Chan VL (2004) FlaC, a protein of Campylobacter jejuni TGH9011 (ATCC43431) secreted through the flagellar apparatus, binds epithelial cells and influences cell invasion. Mol Microbiol 53:541–553
Spiller RC (2007) Role of infection in irritable bowel syndrome. J Gastroenterol 42(Suppl 17):41–47
St Michael F, Szymanski CM, Li J, Chan KH, Khieu NH, Larocque S, Wakarchuk WW, Brisson JR, Monteiro MA (2002) The structures of the lipooligosaccharide and capsule polysaccharide of Campylobacter jejuni genome sequenced strain NCTC 11168. Eur J Biochem 269:5119–5136
Stintzi A (2003) Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J Bacteriol 185:2009–2016
Stintzi A, Marlow D, Palyada K, Naikare H, Panciera R, Whitworth L, Clarke C (2005) Use of genome-wide expression profiling and mutagenesis to study the intestinal lifestyle of Campylobacter jejuni. Infect Immun 73:1797–1810
Svensson SL, Davis LM, MacKichan JK, Allan BJ, Pajaniappan M, Thompson SA, Gaynor EC (2009) The CprS sensor kinase of the zoonotic pathogen Campylobacter jejuni influences biofilm formation and is required for optimal chick colonization. Mol Microbiol 71:253–272
Szymanski CM, Yao R, Ewing CP, Trust TJ, Guerry P (1999) Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol Microbiol 32:1022–1030
Szymanski CM, King M, Haardt M, Armstrong GD (1995) Campylobacter jejuni motility and invasion of Caco-2 cells. Infect Immun 63:4295–4300
Szymanski CM, Burr DH, Guerry P (2002) Campylobacter protein glycosylation affects host cell interactions. Infect Immun 70:2242–2244
Takata T, Fujimoto S, Amako K (1992) Isolation of nonchemotactic mutants of Campylobacter jejuni and their colonization of the mouse intestinal tract. Infect Immun 60:3596–3600
Thibault P, Logan SM, Kelly JF, Brisson JR, Ewing CP, Trust TJ, Guerry P (2001) Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J Biol Chem 276:34862–34870
Tracz DM, Keelan M, Ahmed-Bentley J, Gibreel A, Kowalewska-Grochowska K, Taylor DE (2005) pVir and bloody diarrhea in Campylobacter jejuni enteritis. Emerg Infect Dis 11:838–843
van Alphen LB, Bleumink-Pluym NM, Rochat KD, van Balkom BW, Wosten MM, van Putten JPM (2008a) Active migration into the subcellular space precedes Campylobacter jejuni invasion of epithelial cells. Cell Microbiol 10:53–66
van Alphen LB, Wuhrer M, Bleumink-Pluym NM, Hensbergen PJ, Deelder AM, van Putten JPM (2008b) A functional Campylobacter jejuni maf4 gene results in novel glycoforms on flagellin and altered autoagglutination behaviour. Microbiology 154:3385–3397
Van Rhijn I, Bleumink-Pluym NM, Van Putten JPM, Van den Berg LH (2002) Campylobacter DNA is present in circulating myelomonocytic cells of healthy persons and in persons with Guillain-Barre syndrome. J Infect Dis 185:262–265
van Spreeuwel JP, Duursma GC, Meijer CJ, Bax R, Rosekrans PC, Lindeman J (1985) Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut 26:945–951
Walker RI, Caldwell MB, Lee EC, Guerry P, Trust TJ, Ruiz-Palacios GM (1986) Pathophysiology of Campylobacter enteritis. Microbiol Rev 50:81–94
Walker RI, Schmauder-Chock EA, Parker JL, Burr D (1988) Selective association and transport of Campylobacter jejuni through M cells of rabbit Peyer's patches. Can J Microbiol 34:1142–1147
Wassenaar TM, Bleumink-Pluym NM, van der Zeijst BA (1991) Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J 10:2055–2061
Wassenaar TM, Engelskirchen M, Park S, Lastovica A (1997) Differential uptake and killing potential of Campylobacter jejuni by human peripheral monocytes/macrophages. Med Microbiol Immunol 186:139–144
Wassenaar TM, Fry BN, van der Zeijst BA (1995) Variation of the flagellin gene locus of Campylobacter jejuni by recombination and horizontal gene transfer. Microbiology 141(Pt 1):95–101
Wassenaar TM, Wagenaar JA, Rigter A, Fearnley C, Newell DG, Duim B (2002) Homonucleotide stretches in chromosomal DNA of Campylobacter jejuni display high frequency polymorphism as detected by direct PCR analysis. FEMS Microbiol Lett 212:77–85
Watson RO, Galán JE (2005) Signal transduction in Campylobacter jejuni-induced cytokine production. Cell Microbiol 7:655–665
Watson RO, Galán JE (2008) Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog 4:e14
Watson RO, Novik V, Hofreuter D, Lara-Tejero M, Galán JE (2007) A MyD88-deficient mouse model reveals a role for Nramp1 in Campylobacter jejuni infection. Infect Immun 75:1994–2003
Whitehouse CA, Balbo PB, Pesci EC, Cottle DL, Mirabito PM, Pickett CL (1998) Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun 66:1934–1940
Wooldridge KG, Williams PH, Ketley JM (1996) Host signal transduction and endocytosis of Campylobacter jejuni. Microb Pathog 21:299–305
Wösten MM, van Mourik A, van Putten JPM (2008) Regulation of genes in Campylobacter jejuni. In: Nachamkin I, Szymanski CM, Blaser MJ (eds) Campylobacter. ASM press, Washington, DC, pp 611–624
Wösten MM, Parker CT, van Mourik A, Guilhabert MR, van Dijk L, van Putten JPM (2006) The Campylobacter jejuni PhosS/PhosR operon represents a non-classical phosphate-sensitive two-component system. Mol Microbiol 62:278–291
Wösten MM, Wagenaar JA, van Putten JPM (2004) The FlgS/FlgR two-component signal transduction system regulates the fla regulon in Campylobacter jejuni. J Biol Chem 279:16214–16222
Wright JA, Grant AJ, Hurd D, Harrison M, Guccione EJ, Kelly DJ, Maskell DJ (2009) Metabolite and transcriptome analysis of Campylobacter jejuni in vitro growth reveals a stationary-phase physiological switch. Microbiology 155:80–94
Yao R, Burr DH, Doig P, Trust TJ, Niu H, Guerry P (1994) Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol Microbiol 14:883–893
Yao R, Burr DH, Guerry P (1997) CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol 23:1021–1031
Young NM, Brisson JR, Kelly J, Watson DC, Tessier L, Lanthier PH, Jarrell HC, Cadotte N, St Michael F, Aberg E, Szymanski CM (2002) Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium, Campylobacter jejuni. J Biol Chem 277:42530–42539
Yuki N, Susuki K, Koga M, Nishimoto Y, Odaka M, Hirata K, Taguchi K, Miyatake T, Furukawa K, Kobata T, Yamada M (2004) Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc Natl Acad Sci USA 101:11404–11409
Yun J, Jeon B, Barton YW, Plummer P, Zhang Q, Ryu S (2008) Role of the DksA-like protein in the pathogenesis and diverse metabolic activity of Campylobacter jejuni. J Bacteriol 190:4512–4520
Zheng J, Meng J, Zhao S, Singh R, Song W (2008) Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted cytolethal distending toxin- and Toll-like receptor-mediated activation of NF-kappaB. Infect Immun 76:4498–4508
Zilbauer M, Dorrell N, Boughan PK, Harris A, Wren BW, Klein NJ, Bajaj-Elliott M (2005) Intestinal innate immunity to Campylobacter jejuni results in induction of bactericidal human beta-defensins 2 and 3. Infect Immun 73:7281–7289
Zilbauer M, Dorrell N, Elmi A, Lindley KJ, Schuller S, Jones HE, Klein NJ, Nunez G, Wren BW, Bajaj-Elliott M (2007) A major role for intestinal epithelial nucleotide oligomerization domain 1 (NOD1) in eliciting host bactericidal immune responses to Campylobacter jejuni. Cell Microbiol 9:2404–2416
Acknowledgements
The authors are financially supported by grants from the Netherlands Organization of Health Research and Development (ZonMW-grant 9120-6150 and VIDI-grant 917.66.330).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
van Putten, J.P.M., van Alphen, L.B., Wösten, M.M.S.M., de Zoete, M.R. (2009). Molecular Mechanisms of Campylobacter Infection. In: Sasakawa, C. (eds) Molecular Mechanisms of Bacterial Infection via the Gut. Current Topics in Microbiology and Immunology, vol 337. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-01846-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-01846-6_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-01845-9
Online ISBN: 978-3-642-01846-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)