Abstract
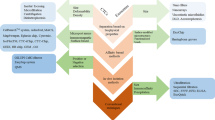
Early cancer screening and effective diagnosis is the most effective form to diminish the number of cancer-related deaths. Liquid biopsy constitutes an attractive alternative to tumor biopsy due to its non-invasive nature and sample accessibility, which permits effective screening and patient monitoring. Within the plethora of biomarkers present in circulation, liquid biopsy has mainly been performed by analyzing circulating tumor cells, and more recently, extracellular vesicles. Tracking these biological particles could provide valuable insights into cancer origin, progression, treatment efficacy, and patient prognosis. Microfluidic devices have emerged as viable solutions for point-of-care cancer screening and monitoring due to their user-friendly operation, low operation costs, and capability of processing, quantifying, and analyzing these bioparticles in a single device. However, the size difference between cells and exosomes (micrometer vs nanometer) requires an adaptation of microfluidic isolation approaches, particularly in label-free methodologies governed by particle and fluid mechanics. This chapter will explore the theory behind particle isolation and sorting in different microfluidic techniques necessary to guide researchers into the design and development of such devices.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Guan X (2015) Cancer metastases: challenges and opportunities. Acta Pharm Sin B 5:402–418
Abdalla TSA, Meiners J, Riethdorf S et al (2021) Prognostic value of preoperative circulating tumor cells counts in patients with UICC stage I-IV colorectal cancer. PLoS One 16:e0252897. https://doi.org/10.1371/journal.pone.0252897
Lee CH, Hsieh JCH, Wu TMH et al (2019) Baseline circulating stem-like cells predict survival in patients with metastatic breast cancer. BMC Cancer 19:1–10. https://doi.org/10.1186/s12885-019-6370-1
Moreno JG, Miller MC, Gross S et al (2005) Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology 65:713–718. https://doi.org/10.1016/j.urology.2004.11.006
Aaltonen KE, Novosadová V, Bendahl PO et al (2017) Molecular characterization of circulating tumor cells from patients with metastatic breast cancer reflects evolutionary changes in gene expression under the pressure of systemic therapy. Oncotarget 8:45544–45565. https://doi.org/10.18632/oncotarget.17271
Bredemeier M, Edimiris P, Tewes M et al (2016) Establishment of a multimarker qPCR panel for the molecular characterization of circulating tumor cells in blood samples of metastatic breast cancer patients during the course of palliative treatment. Oncotarget 7:41677–41690. https://doi.org/10.18632/oncotarget.9528
De Luca F, Rotunno G, Salvianti F et al (2016) Mutational analysis of single circulating tumor cells by next generation sequencing in metastatic breast cancer. Oncotarget 7:26107–26119. https://doi.org/10.18632/oncotarget.8431
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289
Hoshino A, Costa-Silva B, Shen T-L et al (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527:329–335. https://doi.org/10.1038/nature15756
Thakur BK, Zhang H, Becker A et al (2014) Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 24:766–769. https://doi.org/10.1038/cr.2014.44
Xu R, Rai A, Chen M et al (2018) Extracellular vesicles in cancer – implications for future improvements in cancer care. Nat Rev Clin Oncol 15:617–638
Russano M, Napolitano A, Ribelli G et al (2020) Liquid biopsy and tumor heterogeneity in metastatic solid tumors: the potentiality of blood samples. J Exp Clin Cancer Res 39:95. https://doi.org/10.1186/s13046-020-01601-2
Zhou B, Xu K, Zheng X et al (2020) Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther 5:1–14. https://doi.org/10.1038/s41392-020-00258-9
Squires TM, Messinger RJ, Manalis SR (2008) Making it stick: convection, reaction and diffusion in surface-based biosensors. Nat Biotechnol 26:417–426. https://doi.org/10.1038/nbt1388
Dorayappan KDP, Gardner ML, Hisey CL et al (2019) A microfluidic chip enables isolation of exosomes and establishment of their protein profiles and associated signaling pathways in ovarian cancer. Cancer Res 79:3503–3513. https://doi.org/10.1158/0008-5472.CAN-18-3538
Hisey CL, Dorayappan KDP, Cohn DE et al (2018) Microfluidic affinity separation chip for selective capture and release of label-free ovarian cancer exosomes. Lab Chip 18:3144–3153. https://doi.org/10.1039/c8lc00834e
Lo TW, Zhu Z, Purcell E et al (2020) Microfluidic device for high-throughput affinity-based isolation of extracellular vesicles. Lab Chip 20:1762–1770. https://doi.org/10.1039/c9lc01190k
Barriere G, Fici P, Gallerani G et al (2014) Circulating tumor cells and epithelial, mesenchymal and stemness markers: characterization of cell subpopulations. Ann Transl Med 2:109
Cho HY, Choi JH, Lim J et al (2021) Microfluidic chip-based cancer diagnosis and prediction of relapse by detecting circulating tumor cells and circulating cancer stem cells. Cancers (Basel) 13:1–17
Kang YT, Hadlock T, Lo TW et al (2020) Dual-isolation and profiling of circulating tumor cells and cancer exosomes from blood samples with melanoma using Immunoaffinity-based microfluidic interfaces. Adv Sci 7:2001581. https://doi.org/10.1002/advs.202001581
Hou HW, Warkiani ME, Khoo BL et al (2013) Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci Rep 3:1–8. https://doi.org/10.1038/srep01259
Willms E, Cabañas C, Mäger I et al (2018) Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol 9:1. https://doi.org/10.3389/fimmu.2018.00738
Skotland T, Sandvig K, Llorente A (2017) Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res 66:30–41. https://doi.org/10.1016/j.plipres.2017.03.001
Kang YT, Purcell E, Palacios-Rolston C et al (2019) Isolation and profiling of circulating tumor-associated exosomes using extracellular vesicular lipid–protein binding affinity based microfluidic device. Small 15. https://doi.org/10.1002/smll.201903600
Cho H, Kim J, Song H et al (2018) Microfluidic technologies for circulating tumor cell isolation. Analyst 143:2936–2970
Contreras-Naranjo JC, Wu HJ, Ugaz VM (2017) Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip 17:3558–3577
Iliescu FS, Vrtačnik D, Neuzil P, Iliescu C (2019) Microfluidic technology for clinical applications of exosomes. Micromachines 10
Lei KF (2020) A review on microdevices for isolating circulating tumor cells. Micromachines:11
Gou Y, Jia Y, Wang P, Sun C (2018) Progress of inertial microfluidics in principle and application. Sensors (Basel):18
Zhou Y, Ma Z, Tayebi M, Ai Y (2019) Submicron particle focusing and exosome sorting by wavy microchannel structures within viscoelastic fluids. Anal Chem 91:4577–4584. https://doi.org/10.1021/acs.analchem.8b05749
Xiang N, Zhang X, Dai Q et al (2016) Fundamentals of elasto-inertial particle focusing in curved microfluidic channels. Lab Chip 16:2626–2635. https://doi.org/10.1039/c6lc00376a
Tay HM, Kharel S, Dalan R et al (2017) Rapid purification of sub-micrometer particles for enhanced drug release and microvesicles isolation. NPG Asia Mater 9:e434–e434. https://doi.org/10.1038/am.2017.175
Razavi Bazaz S, Mashhadian A, Ehsani A et al (2020) Computational inertial microfluidics: a review. Lab Chip 20:1023–1048
Zhang J, Yan S, Yuan D et al (2016) Fundamentals and applications of inertial microfluidics: a review. Lab Chip 16:10–34
Smith KJ, Jana JA, Kaehr A et al (2021) Inertial focusing of circulating tumor cells in whole blood at high flow rates using the microfluidic CTCKey™ device for CTC enrichment. Lab Chip 21:3559–3572. https://doi.org/10.1039/D1LC00546D
Renier C, Pao E, Che J et al (2017) Label-free isolation of prostate circulating tumor cells using vortex microfluidic technology. npj Precis Oncol 1:15. https://doi.org/10.1038/s41698-017-0015-0
Liu C, Guo J, Tian F et al (2017) Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 11:6968–6976. https://doi.org/10.1021/acsnano.7b02277
Asghari M, Cao X, Mateescu B et al (2020) Oscillatory viscoelastic microfluidics for efficient focusing and separation of nanoscale species. ACS Nano 14:422–433. https://doi.org/10.1021/acsnano.9b06123
Lemaire CA, Liu SZ, Wilkerson CL et al (2018) Fast and label-free isolation of circulating tumor cells from blood: from a research microfluidic platform to an automated fluidic instrument, VTX-1 liquid biopsy system. SLAS Technol 23:16–29. https://doi.org/10.1177/2472630317738698
Guglielmi R, Lai Z, Raba K et al (2020) Technical validation of a new microfluidic device for enrichment of CTCs from large volumes of blood by using buffy coats to mimic diagnostic leukapheresis products. Sci Rep 10:1–9. https://doi.org/10.1038/s41598-020-77227-3
Warkiani ME, Khoo BL, Wu L et al (2016) Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat Protoc 11:134–148. https://doi.org/10.1038/nprot.2016.003
Xu Z, Wang S, Li Y et al (2014) Covalent functionalization of graphene oxide with biocompatible poly(ethylene glycol) for delivery of paclitaxel. ACS Appl Mater Interfaces 6:17268–17276. https://doi.org/10.1021/am505308f
Kaneda Y, Tsutsumi Y, Yoshioka Y et al (2004) The use of PVP as a polymeric carrier to improve the plasma half-life of drugs. Biomaterials 25:3259–3266. https://doi.org/10.1016/j.biomaterials.2003.10.003
Nguyen MK, Lee DS (2010) Bioadhesive PAA-PEG-PAA triblock copolymer hydrogels for drug delivery in oral cavity. Macromol Res 18:284–288. https://doi.org/10.1007/s13233-010-0315-5
Dehghani M, Lucas K, Flax J et al (2019) Tangential flow microfluidics for the capture and release of nanoparticles and extracellular vesicles on conventional and ultrathin membranes. Adv Mater Technol 4:1900539. https://doi.org/10.1002/admt.201900539
Chen K, Amontree J, Varillas J et al (2020) Incorporation of lateral microfiltration with immunoaffinity for enhancing the capture efficiency of rare cells. Sci Rep 10:14210. https://doi.org/10.1038/s41598-020-71041-7
Lyklema J (2005) Fundamentals of interface and colloid science, 1st edn. Elsevier
Chen Z, Yang Y, Yamaguchi H et al (2020) Isolation of cancer-derived extracellular vesicle subpopulations by a size-selective microfluidic platform. Biomicrofluidics 14. https://doi.org/10.1063/5.0008438
Cho S, Jo W, Heo Y et al (2016) Isolation of extracellular vesicle from blood plasma using electrophoretic migration through porous membrane. Sens Actuators B 233:289–297. https://doi.org/10.1016/j.snb.2016.04.091
Davies RT, Kim J, Jang SC et al (2012) Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip 12:5202–5210. https://doi.org/10.1039/c2lc41006k
Shilton RJ, Travagliati M, Beltram F, Cecchini M (2014) Nanoliter-droplet acoustic streaming via ultra high frequency surface acoustic waves. Adv Mater 26:4941–4946. https://doi.org/10.1002/adma.201400091
Liu H, Ao Z, Cai B et al (2018) Size-amplified acoustofluidic separation of circulating tumor cells with removable microbeads. Nano Futur 2:025004. https://doi.org/10.1088/2399-1984/aabf50
Wu Z, Jiang H, Zhang L et al (2019) The acoustofluidic focusing and separation of rare tumor cells using transparent lithium niobate transducers. Lab Chip 19:3922–3930. https://doi.org/10.1039/c9lc00874h
Li S, Ma F, Bachman H et al (2017) Acoustofluidic bacteria separation. J Micromech Microeng 27:015031. https://doi.org/10.1088/1361-6439/27/1/015031
Lee K, Shao H, Weissleder R, Lee H (2015) Acoustic purification of extracellular microvesicles. ACS Nano 9:2321–2327. https://doi.org/10.1021/nn506538f
Wu M, Ouyang Y, Wang Z et al (2017) Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A 114:10584–10589. https://doi.org/10.1073/pnas.1709210114
Inglis DW, Davis JA, Austin RH, Sturm JC (2006) Critical particle size for fractionation by deterministic lateral displacement. Lab Chip 6:655–658. https://doi.org/10.1039/b515371a
Wunsch BH, Smith JT, Gifford SM et al (2016) Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20nm. Nat Nanotechnol 11:936–940. https://doi.org/10.1038/nnano.2016.134
Liu Z, Huang F, Du J et al (2013) Rapid isolation of cancer cells using microfluidic deterministic lateral displacement structure. Biomicrofluidics 7. https://doi.org/10.1063/1.4774308
Liu Z, Zhang W, Huang F et al (2013) High throughput capture of circulating tumor cells using an integrated microfluidic system. Biosens Bioelectron 47:113–119. https://doi.org/10.1016/j.bios.2013.03.017
Liu Z, Huang Y, Liang W et al (2021) Cascaded filter deterministic lateral displacement microchips for isolation and molecular analysis of circulating tumor cells and fusion cells. Lab Chip 21:2881–2891. https://doi.org/10.1039/D1LC00360G
Zhao W, Liu Y, Jenkins BD et al (2019) Tumor antigen-independent and cell size variation-inclusive enrichment of viable circulating tumor cells. Lab Chip 19:1860–1876. https://doi.org/10.1039/c9lc00210c
Zeming KK, Thakor NV, Zhang Y, Chen CH (2016) Real-time modulated nanoparticle separation with an ultra-large dynamic range. Lab Chip 16:75–85. https://doi.org/10.1039/c5lc01051a
Liu Y, Zhao W, Cheng R et al (2020) Label-free ferrohydrodynamic separation of exosome-like nanoparticles. Lab Chip 20:3187–3201. https://doi.org/10.1039/d0lc00609b
Zhao W, Cheng R, Jenkins BD et al (2017) Label-free ferrohydrodynamic cell separation of circulating tumor cells. Lab Chip 17:3097–3111. https://doi.org/10.1039/c7lc00680b
Acknowledgements
D.C. acknowledges the financial support from the Portuguese Foundation for Science and Technology (FCT) under the program CEEC Individual 2017 (CEECIND/00352/2017). C.M.A, S.C.K, and D.C. also thank the support from the FCT under the scope of the projects 2MATCH (PTDC/BTM-ORG/28070/2017) and BREAST-IT (PTDC/BTM-ORG/28168/2017) funded by the Programa Operacional Regional do Norte supported by European Regional Development Funds (ERDF). The authors also thank the financial support from the European Union Framework Program for Research and Innovation Horizon 2020 on the FoReCaST project under (Grant Number: 668983).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
None.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Abreu, C.M., Caballero, D., Kundu, S.C., Reis, R.L. (2022). From Exosomes to Circulating Tumor Cells: Using Microfluidics to Detect High Predictive Cancer Biomarkers. In: Caballero, D., Kundu, S.C., Reis, R.L. (eds) Microfluidics and Biosensors in Cancer Research. Advances in Experimental Medicine and Biology, vol 1379. Springer, Cham. https://doi.org/10.1007/978-3-031-04039-9_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-04039-9_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04038-2
Online ISBN: 978-3-031-04039-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)