Abstract
Approaches for the collection and analysis of plastic debris in environmental matrices are rapidly evolving. Such plastics span a continuum of sizes, encompassing large (macro-), medium (micro-, typically defined as particles between 1 μm and 5 mm), and smaller (nano-) plastics. All are of environmental relevance. Particle sizes are dynamic. Large plastics may fragment over time, while smaller particles may agglomerate in the field. The diverse morphologies (fragment, fiber, sphere) and chemical compositions of microplastics further complicate their characterization. Fibers are of growing interest and present particular analytical challenges due to their narrow profiles. Compositional classes of emerging concern include tire wear, paint chips, semisynthetics (e.g., rayon), and bioplastics. Plastics commonly contain chemical additives and fillers, which may alter their toxicological potency, behavior (e.g., buoyancy), or detector response (e.g., yield fluorescence) during analysis. Field sampling methods often focus on >20 μm and even >300 μm sized particles and will thus not capture smaller microplastics (which may be most abundant and bioavailable). Analysis of a limited subgroup (selected polymer types, particle sizes, or shapes) of microplastics, while often operationally necessary, can result in an underestimation of actual sample content. These shortcomings complicate calls for toxicological studies of microplastics to be based on “environmentally relevant concentrations.” Sample matrices of interest include water (including wastewater, ice, snow), sediment (soil, dust, wastewater sludge), air, and biota. Properties of the environment, and of the particles themselves, may concentrate plastic debris in select zones (e.g., gyres, shorelines, polar ice, wastewater sludge). Sampling designs should consider such patchy distributions. Episodic releases due to weather and anthropogenic discharges should also be considered. While water grab samples and sieving are commonplace, novel techniques for microplastic isolation, such as continuous flow centrifugation, show promise. The abundance of nonplastic particulates (e.g., clay, detritus, biological material) in samples interferes with microplastic detection and characterization. Their removal is typically accomplished using a combination of gravity separation and oxidative digestion (including strong bases, peroxide, enzymes); unfortunately, aggressive treatments may damage more labile plastics. Microscope-based infrared or Raman detection is often applied to provide polymer chemistry and morphological data for individual microplastic particles. However, the sheer number of particles in many samples presents logistical hurdles. In response, instruments have been developed that employ detector arrays and rapid scanning lasers. The addition of dyes to stain particulates may facilitate spectroscopic detection of some polymer types. Most researchers provide microplastic data in the form of the abundances of polymer types within particle size, polymer, and morphology classes. Polymer mass data in samples remain rare but are essential to elucidating fate. Rather than characterizing individual particles in samples, solvent extraction (following initial sample prep, such as sediment size class sorting), combined with techniques such as thermoanalysis (e.g., pyrolysis), has been used to generate microplastic mass data. However, this may obviate the acquisition of individual particle morphology and compositional information. Alternatively, some techniques (e.g., electron and atomic force microscopy and matrix-assisted laser desorption mass spectrometry) are adept at providing highly detailed data on the size, morphology, composition, and surface chemistry of select particles. Ultimately, the analyst must select the approach best suited for their study goals. Robust quality control elements are also critical to evaluate the accuracy and precision of the sampling and analysis techniques. Further, improved efforts are required to assess and control possible sample contamination due to the ubiquitous distribution of microplastics, especially in indoor environments where samples are processed.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Abbreviations- ABS:
-
Acrylonitrile butadiene styrene
- AFM:
-
Atomic force microscopy
- APPI:
-
Atmospheric pressure photoionization
- ATR:
-
Attenuated total reflectance
- BPA:
-
Bisphenol A
- DART:
-
Direct analysis in real time
- DCM:
-
Dichloromethane (methylene chloride)
- DESI:
-
Desorption electrospray ionization
- EA/IRMS:
-
Elemental analyzer/isotope ratio mass spectrometry
- EDS:
-
Energy-dispersive X-ray spectroscopy
- EM :
-
Electron microscopy
- ESCA:
-
Electron spectroscopy for chemical analysis
- EVA:
-
Ethylene vinyl acetate
- FM:
-
Fluorescence microscopy
- FPA:
-
Focal plane array
- FR:
-
Flame retardant
- FTIR :
-
Fourier transform infrared spectroscopy
- GC:
-
Gas chromatography
- GPC:
-
Gel permeation chromatography
- HDPE:
-
High-density polyethylene
- HRMS:
-
High-resolution mass spectrometry
- IR:
-
Infrared (spectroscopy)
- LC:
-
Liquid chromatography
- LC-MS/MS:
-
Liquid chromatography/tandem mass spectrometry
- LDPE:
-
Low-density polyethylene
- LOD :
-
Limit of detection
- m/z :
-
Mass-to-charge ratio
- MALDI:
-
Matrix-assisted laser desorption/ionization
- MP:
-
Microplastic
- MPSS:
-
Munich plastic sediment separator
- MS:
-
Mass spectrometry
- MW:
-
Molecular weight
- NP:
-
Nanoplastic
- NR:
-
Nile red
- O-PTIR:
-
Optical photothermal IR
- PA:
-
Polyamide
- PC:
-
Polycarbonate
- PE:
-
Polyethylene
- PET:
-
Poly(ethylene terephthalate)
- PP:
-
Polypropylene
- PS :
-
Polystyrene
- PU:
-
Polyurethane
- PVC:
-
Polyvinyl chloride
- Py-GC/MS :
-
Pyrolysis-gas chromatography/mass spectrometry
- QA:
-
Quality assurance
- QC:
-
Quality control
- Q-TOF:
-
Quadrupole time of flight
- rpm:
-
Revolutions per minute
- RT:
-
Room temperature
- SEC :
-
Size exclusion chromatography
- SEM :
-
Scanning electron microscopy
- SFC:
-
Supercritical fluid chromatography
- TD-PTR-MS:
-
Thermal desorption-proton transfer reaction-mass spectrometry
- TED-GC/MS:
-
Thermal extraction desorption-gas chromatography/mass spectrometry
- TEM :
-
Transmission electron microscopy
- TGA:
-
Thermogravimetric analysis
- TMAH:
-
Tetramethylammonium hydroxide
- TOF:
-
Time of flight
- TOF-SIMS:
-
Time-of-flight secondary ion mass spectrometry
- UHMW:
-
Ultrahigh molecular weight
- UHPLC:
-
Ultrahigh performance liquid chromatography
- XPS:
-
X-ray photoelectron spectroscopy
- μFTIR :
-
Micro-Fourier transform infrared spectroscopy
- μRaman:
-
Micro-Raman spectroscopy
2.1 Introduction
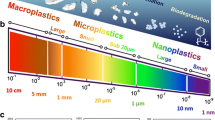
To date, the lack of sampling and analytical methods capable of adequately characterizing the diversity of plastic debris in the environment has handicapped studies of their distribution, fate, and consequences. Plastic debris in the environment exists in a continuum of sizes. Debris has been classified as macro- (>25 mm), meso- (5–25 mm), micro- (1 μm to 5 mm), and nanoplastic (<1 μm). Where not differentiated, the use of the term “microplastics” here will mean all particles <5 mm. In the environment, plastics fragment over time, rates varying depending on polymer composition and ambient conditions. As such, size distributions are not static. Most published methods have been designed to detect only a subset of microplastics (often those > 300 μm). Hence, resulting measurements are likely underestimates. Commonly, the number of particles detected in a sample (within a size range) or the identities of only select polymer types are reported, versus the complete plastic mass-based concentration. Readers should take these limitations into account when interpreting published studies. Plastics in the environment exhibit a range of properties and composition.
Representative sampling followed by comprehensive, accurate analysis of microplastics is a prerequisite for understanding their fate and biological consequences and for crafting effective solutions. When developing and applying methodologies, researchers must carefully consider study goals (Fig. 2.1). Both field and controlled (lab or mesocosm) approaches are needed to answer important questions. Controlled experiments typically utilize specific, preselected test plastics. A good understanding of plastic composition and properties is essential. Also, while the majority of lab studies employ un-weathered materials, plastics start to be altered once in use and following environmental release. The extent of weathering is a function of ambient conditions and duration, adding further complexity and variability to the microplastics to be analyzed (Luo et al. 2019, 2020; Zhang et al. 2021).
Researchers must first delineate their study goals and then select appropriate sampling and analysis approaches. For example, focusing on the detailed characteristics (e.g., size, shape, texture, composition, extent of weathering) of a few 10 μm microplastic particles (via, e.g., MALDI-MS) is informative (represented by the microscope icon). However, such a narrowly defined focus may not be compatible with a goal of assessing the range of diverse microplastics in, for example, an entire forested area. Further, ignoring large debris in favor of microplastics alone is problematic as the former will eventually degrade into many small fragments. Microplastics present at the time of sampling represent a snapshot of a dynamic situation. Further, remedies such as removal (and better prevention) of large plastic debris are critical. Documentation and removal of large debris also can be performed by those lacking sophisticated analytical tools, such as “citizen scientists.” (Photo: Alaskan forest floor adjacent to a marine shoreline. Credit: Ted Raynor, GoAK.org)
In studies of field-collected samples, the analytical methods typically applied do not encompass the complete range of plastic characteristics (e.g., polymer type, size, morphology). Polymers targeted are generally those manufactured in greatest abundance or commonly reported in surveys, e.g., those in single-use containers, such as polyethylene (PE), poly(ethylene terephthalate) (PET), and polystyrene (PS). This is comparable to prioritizing high production volume chemicals for monitoring, without factoring in their relative risks or potential to alter ecosystems. To elaborate, single-use beverage containers are dominant plastic debris components. However, microplastics (mostly PE, polypropylene (PP), and PET) generated from these may exhibit modest chemical risk (Lithner et al. 2011), as these products were designed to present minimal threats to human health. In contrast, e-waste plastics typically contain percent concentrations by weight of persistent and toxic additives (Singh et al. 2020), such as flame retardants (Li et al. 2019) and metals (e.g., Cd, Cr, Hg, Pb, and Sb) at levels that may exceed hazardous waste guidelines (Turner et al. 2019). Hence, from an ecosystem health perspective, less abundant plastic products might be disproportionately impactful and worthy of prioritization for analysis.
Some have criticized laboratory-based studies for the use of “unrealistically” high microplastic concentrations. However, if existing measurements do not adequately represent the true levels present in the environment, this pronouncement may be hasty (Hale 2018; Covernton et al. 2019). In addition, environmental burdens are increasing at exponential rates, with an estimated doubling rate in, for example, coastal marine sediments of 15 years (Brandon et al. 2019). The quantification of microplastics in surface waters further illustrates this point. Most approaches to date have deployed sampling gear (e.g., plankton nets) with openings exceeding 300 μm. Thus, smaller particles may not be retained. Smaller microplastics are more difficult to detect but ironically may be more abundant in environmental samples (e.g., Enders et al. 2015). They may also present heightened toxicological impacts (von Moos et al. 2012; Kögel et al. 2020) due to their ability to infiltrate tissues (e.g., lung alveoli in mammals) and penetrate cell membranes (Prata et al. 2020). Small microplastics and nanoplastics also exhibit exaggerated surface areas and thus enhanced capacity for environmental interactions, including contaminant sorption (Wang et al. 2019).
Spatially, most published monitoring has focused on microplastics at the water’s surface. Recently, interests in denser polymers and fibers and debris at depth have emerged. Studies on microplastics in air (Gasperi et al. 2018), soils (Ng et al. 2018), and sediments (Gomiero et al. 2019) are appearing in growing numbers. For example, Choy et al. (2019) observed in vertical transects off Monterey Bay, California, greater microplastic water concentrations between 200 and 600 m than at the surface. They reported that weathered PET and polyamide (PA) fibers (negatively buoyant polymers) dominated. Kane et al. (2020) reported up to 1.9 million microplastics (primarily fibers) m−2 in deepwater, sedimentary drift deposits in the Tyrrhenian Sea. Yu et al. (2018) observed that PET and cellulose-derived fibers were the major forms on southeastern US coastal beaches. Fibers present additional sampling and detection challenges due to their elongated shapes and small cross sections. Hence, analytical methods must be refined to accommodate these.
Additional subclasses of microplastics merit scrutiny. Paint chips and tire wear fragments have been less studied but are reported to be major components of microplastic debris in some environmental samples (Hale et al. 2020). These present novel analytical issues and will be discussed later in greater detail. Paint chips have been observed to be abundant in surface waters with substantial boat traffic (e.g., Imhof et al. 2016), as well as near shipyards (Turner 2010). Chips often exhibit distinct colors, facilitating visual identification. However, they may be quite small and contain high concentrations of additives that can confound spectra often used for polymer identification. Pigments can contain toxic organic or metallic compounds, so their identification and health consequences should be assessed (Turner 2010; Luo et al. 2020). Importantly, the ecological repercussions of natural particles (e.g., cellulose, chitin, and minerals) and processed bio-based (e.g., cellulose acetate, polylactic acid) versus fossil fuel-based plastics merit further evaluation. Their determination adds an additional layer of analytical considerations.
Recent reviews of microplastic analysis techniques have been published (e.g., Hidalgo-Ruz et al. 2012; Löder and Gerdts 2015; Van Cauwenberghe et al. 2015; Lusher et al. 2017; GESAMP 2019; Fu et al. 2020). Our goals in the subsequent sections of this chapter will be to present representative accepted, as well as some more novel approaches, and to describe challenges and conceptual elements.
2.2 About the Analytes
Being complex solids composed primarily of high molecular weight and low volatility polymers, sampling and analytical considerations for plastic debris diverge from those of more commonly monitored lower molecular weight contaminants, such as pesticides or metals. The latter are amenable to well-established and widely available techniques, such as gas chromatography/mass spectrometry (GC/MS) and atomic absorption/emission spectroscopy. For plastics, additional characteristics of interest exist, including polymer composition, particle shape, and size. The immense diversity of plastic products in commerce, and thus in the environment, makes their determination challenging (Hale 2017; Rochman et al. 2019). Weathering and abrasion during use and following discard may alter size distributions. Even in the lab, plastic fragmentation can occur. For example, Dawson et al. (2018) observed the generation of nanoplastics by the stomach and gastric mill of Antarctic krill that were fed a defined size class of microplastics in the lab. Aggressive treatment of embrittled microplastics during sample preparation may also affect size distributions.
Plastic composition is an essential factor when choosing sampling and analysis methods as it dictates their fate/behavior in the field and during preparation. Polymers are composed of repeating units or monomers. Plastics may also be composites (e.g., reinforced with fibers) or copolymers (mixture of different polymers). Polymer chains can differ in molecular weight within the same plastic . Chains may be compositionally homogeneous, i.e., consist of the same, or different monomers. The polymeric chains can be composed of, arranged, and chemically linked in various ways. The resulting materials may be amorphous or crystalline, which affects their properties. Residual monomers, as well as catalysts used in synthesis, may be retained in plastic products, adding heterogeneity. Plastics are often infused with additives to achieve the desired color, flame retardancy, flexibility, or other characteristics (Hahladakis et al. 2018). Additive levels, at times reaching percent by weight levels, can complicate the analysis of the plastics (Lenz et al. 2015) and alter their environmental fate and behavior. Fillers (e.g., calcium carbonate, clay, talc, carbon black) may also be incorporated to modify properties or reduce costs and may interfere with the spectroscopic analysis. Tires are an example of a complex product, consisting of natural or synthetic rubbers, polymeric and metallic fibers, carbon black, and a host of additives. Such materials may confound commonly applied identification techniques such as IR spectroscopy.
Once in the environment, plastic debris chemical composition may be modified by weathering, complicating analysis. For example, photooxidation can alter spectroscopic results by increasing the relative carbonyl to methylene absorbance of both polymers and additives (Su et al. 2019; Khaled et al. 2018). After release, debris from diverse sources will intermingle, creating complex heterogeneous mixtures.
Polymer type, form, and additive content affect physical behavior and toxicological outcomes. For example, Luan et al. (2019) reported that certain functional groups on PS resulted in differing effects during the key development stages of the clam Meretrix meretrix. Luo et al. (2019) attributed fluorescent additives, leached from polyurethane (PU) microplastics, to effects on microalgal photosynthesis. Hence, composition is important to determine analytically.
To date, the analysis of additives in plastic debris has been limited (e.g., Hermabessiere et al. 2017). However, extensive work has been done related to additive migration from packaging to food (Hahladakis et al. 2018). Considerable interest has also arisen regarding environmental contaminants that are polymer additives, e.g., flame retardants, in indoor dust and subsequent human exposure (Wu et al. 2007). Analysis of additives is important, but a detailed discussion is beyond the scope of this chapter.
2.3 Sampling
Depending upon study goals, a variety of environmental matrices have been chosen for sampling, for example, water, sediments/soils/dust, air, marine snow, plankton, and specific tissues of larger organisms (e.g., digestive tissues, gills, liver, muscle, etc.).
2.3.1 Aqueous Matrices
Such samples may include natural surface or drinking water, wastewater (influent, in process or effluent), or precipitation (rain, melted snow, or ice). For natural waters, the surface microlayer, water column, and sediment interstitial water may be of particular interest. Each type presents different challenges due to collection requirements and the level of matrix interferences, as well as the abundance and characteristics of the plastics therein. Historically, surface water has been most commonly evaluated due to ease of collection and the presumption that most plastics are buoyant. Recently, data showing substantial microplastics in other environmental compartments have been published (Kooi et al. 2017; Erni-Cassola et al. 2019). Knowledge of site characteristics, such as weather, season and flow patterns, and basin morphology, are critical to designing appropriate sampling and interpreting results.
Method selection criteria include their ability to retain and quantify the salient range of particle sizes (and shapes) and should be evaluated by the analyst (Koelmans et al. 2019). In their global review of small floating plastic debris, van Sebille et al. (2015) estimated that >90% of the surface water trawls contained meshes >330 μm. Hence, the sample particle distributions will differ from those in the field (Dai et al. 2018). Approaches that exclude small microplastics will underestimate the total abundances present (Pabortsava and Lampitt 2020; Covernton et al. 2019). For example, Dris et al. (2018) reported a 250-fold increase in fiber counts when sampling with an 80 μm versus a 330 μm mesh net. Collection methods may perform well for spherical microplastics but poorly for elongated fibers or fragments. Fibers can pass more readily through the mesh, depending on the angle of contact. Changes in particle collection efficiency over time due to blockage of openings can also occur (Prata et al. 2019).
Grab sampling of water may be employed to capture smaller particles, e.g., using buckets for surface and Niskin, Van Dorn, or other remote capture devices at depth. In a novel study, Choy et al. (2019) used a remotely operated vehicle equipped with in situ samplers that pumped water (ranging from 1007 to 2378 m3) through 100 μm mesh filters at selected depths up to 1000 m. Determination of coincident water characteristics (e.g., temperature, salinity, suspended solids, chlorophyll content) may also aid in interpreting microplastic results. Pumping water through a series of sieve(s) or meshes has also been explored (Prata et al. 2019), permitting larger, composite samples to be evaluated. Tamminga et al. (2019) observed orders of magnitude higher numbers of microplastics and more efficient collection of fibers by passing water through a cascade of filters compared to the collection with a manta net. However, comparatively rare debris may be missed due to the smaller volume of water sampled by pump or grab approaches versus towing nets across wide areas. For sampling surface microlayer microplastics, Ng and Obbard (2006) used a rotating drum while Song et al. (2014) sampled via a dipped mesh screen.
While continuous flow centrifugation (CFC) has been widely used for the sampling of suspended particulate matter, to date it has seen limited usage for the collection of microplastics. However, Leslie et al. (2017) collected and concentrated suspended riverine particulate matter by CFC and processed pooled concentrates using salt-based density separation techniques. They noted most microplastics in the suspended particulate matter were <300 μm. Hildebrandt et al. (2020) demonstrated CFC in the lab for the collection and pre-concentration of Pd-doped nanoplastics from ultrapure water and filtered and unfiltered Elbe River (Germany) water. One versus two centrifuges in series and various water flow rates were evaluated. They noted the possibility of removing high-density minerals from suspensions, as well as separating micro- from nanoplastics. Shipboard sampling and passage through the centrifuges would eliminate the need for storage containers, resultant nanoplastic sedimentation/surface adhesion losses, and reduced contamination potential. Compared to filter-based systems, CFC can be run continuously for days, allowing large volumes to be processed.
2.3.2 Air Samples
Microplastics in air are an emerging concern. Outdoors, airborne microplastics can be rapidly transported long distances. Indoors, human exposure via microplastic inhalation and ingestion may be particularly important due to the confined space, abundance of plastic products therein, and low air turnover. Citizens of developed countries often spend >90% of their time indoors. While data pertaining to microplastics remain limited, there is substantial literature on ambient particles in indoor and outdoor air (e.g., Whalley and Zandi 2016). These are typically collected on filters of varying porosity (Zhang et al. 2020a). Note that glass fiber filters are often used here and this matrix may enmesh and obscure microplastics, complicating later spectroscopic evaluation.
Dry and wet deposition of microplastics was recently evaluated by Brahney et al. (2020) in several remote US wilderness areas by initial collection in buckets. Wet samples were subsequently filtered through 0.45 μm polyethersulfone filters and dry material reacquired using a ceramic blade. Stationary high volume, portable personal, and passive samplers may also be used. Sommer et al. (2018) used a Sigma-2 passive sampler to collect airborne particulates near three German roadways. Particles were collected on a transparent adhesive acceptor surface, over 7 days. As in the case of water strata, the collection of air samples at different heights may yield particles of different characteristics (Quang et al. 2012). Akin to growing concerns over the toxicological consequences of small microplastics in water, inhalation of fine airborne particulate matter <10 μm (PM10) has long been recognized as a serious health concern due to its ability to infiltrate lung alveoli (e.g., Anderson et al. 2012). Approaches such as the breathing thermal manikin have been developed in an attempt to mimic human exposure (Vianello et al. 2019).
2.3.3 Sediments, Soils, and Dust
Microplastic contents of these matrices are of increasing concern. Bedded sediments are typically collected as a core or grab. Sediments integrate conditions over extended periods compared to surface water samples, but burdens can vary over short distances. Study goals drive the location and number of discrete samples. Sampling depth is a consideration for cores. As plastics have only become prevalent in the environment since the 1950s, investigations of distributions in sediment cores are rare. However, Brandon et al. (2019) reported plastic debris in a core from the Santa Barbara Basin spanning the period 1834–2009. These authors were limited to larger debris as visual sorting of candidate microplastics was utilized, followed by FTIR polymer identification of selected targets. The authors, after correcting for sample contamination, reported an exponential increase in plastic deposition from 1945 to 2009, with a doubling time of 15 years. Sediments were passed through a 104 μm mesh, so true microplastic concentrations were likely higher. In contrast to most surface water investigations, they also noted that fibers were the dominant form detected in their sediments.
Microplastic loads and particle characteristics vary widely in different sub-environments. For example, Haave et al. (2019) found that distributions of small (<100 μm) and large microplastics (>500 μm) in sediments of a Norwegian urban fjord differed spatially, with small microplastics preferentially observed in areas of higher organic matter deposition. In another example, Ceccarini et al. (2018) collected materials on a transect from subtidal sediments to supralittoral sand. They found large plastic fragments accumulated above the storm berm and higher-density polymer particles in the benthic sediments. Their work underscores the need for techniques that can generate results for the total amount of plastics present (inclusive of sizes normally below the limits of spectroscopic detection of discrete particles) in a sample. Other solids, such as municipal wastewater sludge, are increasingly being examined. Due to the surface skimming and sedimentation processes utilized, treatment sludges may contain >90% of microplastics that enter wastewater facilities (Mahon et al. 2017).
As plastics are primarily manufactured, used, and discarded on land (Hale et al. 2020), the soil is an important media to examine. However, compared to aquatic sediments, terrestrial soils have been less frequently considered. Möller et al. (2020) reviewed available soil sampling and microplastics analysis methods. The former included the use of stainless-steel scoops or shovels for surface samples and cores for samples at depth. They also emphasized choosing a sampling strategy (e.g., judgmental, random, grid, transect, or stratified) consistent with the study goals.
2.3.4 Biological Samples
The diversity of biological organisms is immense, and sampling will depend greatly on study objectives. More variables are in play for biological compared with abiotic media. Stationary organisms will better reflect local conditions than mobile/migratory species. Some organisms may preferentially ingest specific particle sizes (Ward et al. 2019). Small organisms may be composited (e.g., collected onto filters), while larger specimens may be collected (using nets, traps, or hook and line) and analyzed individually, in their entirety, or dissected. An important consideration is whether the microplastics reside within tissues proper or are associated with external or internal (e.g., digestive tract) surfaces. If not within tissues proper, toxicological risks may be less. Microplastics within digestive systems may pass through the body and be depurated. To remove digestive tract-entrained microplastics, the organism may be allowed to depurate gut contents, or the digestive tract manually flushed or removed in the lab. Disposition of microplastics within organisms and mode of meal preparation may also alter the likelihood of subsequent human exposure via ingestion, i.e., if the organism is first depurated, eaten in its entirety (e.g., many shellfish), or otherwise prepared (e.g., filleted finfish). Food preparation often differs regionally and between ethnic groups.
2.3.5 Sample Preservation
Preservation of microplastic samples is not commonly described, in part as most plastics are resistant to biodegradation. However, Courtene-Jones et al. (2017) examined freezing versus formaldehyde/ethanol preservation of microplastics in mussel tissue, reporting no differential effects of these treatments. While most plastics are recalcitrant, coincident sample constituents may be susceptible to decomposition, especially biological tissues. Hence, the lack of preservation may alter the concentration calculation, as the matrix weight is normally used in the denominator for sediments and tissues. Microplastic-containing samples are often held in oxidizing agents for extended periods of time as part of the purification process. For example, Song et al. (2014) digested solids, sieved from surface waters, with 34.5% H2O2 for 2 weeks. Semisynthetic polymers (manufactured from natural precursors) may be more labile and degraded. Common products generated from natural materials include cellophane packaging, cigarette filters, and rayon-based textiles. Cellulose acetate was reported to represent >50% of synthetic particles in landfill leachates (Su et al. 2019), deep-sea sediments (Lusher et al. 2013), ice cores (Obbard et al. 2014), and fish (Lusher et al. 2013).
2.4 Laboratory Processing
As a result of environmental weathering, field sampling, and lab preparation, physical changes in plastic debris may occur due to abrasion with instruments, sieves or sand grains, or sample freeze/thaw cycles (Klein et al. 2018). Biofilm formation and electrostatic interactions on surfaces facilitate agglomeration of microplastics, altering their apparent size and behavior in the environment and during collection and analysis (Rummel et al. 2017; Michels et al. 2018; Lapointe et al. 2020). Depending on matrix complexity, a sequence of preparative steps is typically employed, commonly organic matter digestion and density-based separation. Steps can be divided into sample preparation, microplastic concentration, matrix purification, microplastic size separation, and particle detection (Fig. 2.2). Some methods focus on evaluating the characteristics of individual particles (e.g., those applying vibrational spectroscopic techniques such as Raman or IR spectroscopy), while others focus on the bulk, weight-based concentration of polymers present in the sample (e.g., pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS)) or solvent extraction. As such, sample preparation may be dictated by the characteristics of the detection technique (discussed later).
Possible strategies described in the literature for the analysis of plastic debris in sediment and water samples, from sampling to reporting of the results. The sample preparation here is split into the pretreatment, density separation, and the posttreatment of microplastics. Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy energy-dispersive X-ray spectroscopy (SEM-EDS), pyrolysis- or thermal desorption-gas chromatography/mass spectrometry (Py-GC/MS, TDS-GC/MS), and others may be deployed for the plastic analysis. When ASE separation is utilized, chromatographic analysis techniques not listed here, as well as spectrometric identification, may also be employed. (From Klein et al. (2018). http://creativecommons.org/licenses/by/4.0/. Used with minor editing of the original figure legend)
2.4.1 Sample Preparation
Researchers may process constituents into particle size classes (so-called binning) by passage through a series of increasingly fine sieves or filters. This is especially useful if the ultimate detection approach does not yield individual particle characteristics (e.g., Py-GC/MS). For example, Gomiero et al. (2019) separated microplastics by sequential passage through 250, 100, 40, and 10 μm stainless-steel sieves. The samples had previously been subjected to oxidative and enzymatic cleanup, as well as density-based separation steps (discussed below). Bulk separation/characterization techniques (e.g., solvent extraction, followed by spectroscopy or thermogravimetry) may provide an estimate of total microplastics that encompass contributions from particles smaller than what even sophisticated analytical instruments can detect (typically 10–20 μm, Raman down to 1 μm). Notably, Gomiero et al. (2019) reported that the 40–100 μm fraction, a size range below which is often reported in the literature, contributed most to the total polymer quantity of their field samples. It should be noted that in that study, the field-collected sediments were initially homogenized before further treatment in “a standard stainless-steel orbital mixer (approx. 20 rpm, 10 min at RT) using the K-beater knife.” The sediments were predominantly fine sand. Plastics in the environment may be embrittled by weathering, such as ultraviolet (UV) oxidation (Song et al. 2017; Khaled et al. 2018). Hence, it is possible that abrasive lab homogenization techniques may further fragment brittle microplastics. To their credit, Gomiero et al. (2019) performed a series of procedural validations using spiked unweathered microspheres of three sizes and polymer types (PE, PP, and polyvinyl chloride, PVC). But such polymer types are less vulnerable to alteration by caustic treatments than PA and polycarbonate (PC). In addition, microspheres may be size-fractionated more consistently and be less vulnerable to fragmentation than plastic films or fibers. Further supporting the above concern about abrasive handling fragmenting plastic debris, Efimova et al. (2018) used a laboratory rotating mixer and coarse beach sediment to intentionally generate secondary microplastics in the laboratory.
2.4.2 Chemical and Enzymatic Digestion
Field samples often contain inorganic (e.g., clay minerals) and organic particles (e.g., detritus) that can interfere with the plastic analysis. The removal requirements for such particles vary, depending on the matrix (e.g., water, tissue, or sediments). Plastic debris from the field quickly accumulates an organic coating that may alter its chemical composition, properties (McGivney et al. 2020), and behavior (e.g., promoting aggregation or increasing their apparent density) or confound later spectroscopic analysis. A host of chemical agents have been employed to eliminate such films. Hydrogen peroxide (at different concentrations and temperatures) has been commonly used to oxidize labile organics. Duration of contact varies from hours to days. Some researchers have utilized Fenton’s reagent (a solution of H2O2 and Fe2+; Tagg et al. 2017). Alternatively, acids (e.g., HCl, HNO3, formic) and bases (e.g., NaOH, KOH) have been employed, especially for digesting biological tissues. Repeated treatments and concurrent heating may be required to fully oxidize labile organic matter. However, PA and ester-based polymers (e.g., PET) appear more vulnerable to degradation by these treatments (Karami et al. 2017; Hurley et al. 2018). Wolff et al. (2019) reported substantial alterations of ethylene vinyl acetate (EVA), PU, and PA after exposure to treatment with H2O2, NaClO, ZnCl2, and hexane. Hence, purification methods should initially be validated for the targeted polymer types.
Enzymatic digestion has also been applied to samples. Enzymes typically cause less polymer degradation than caustic agents. Unfortunately, such procedures can be complex (e.g., including detergents, proteases, lipases, chitinases, and cellulases), time-consuming (>10 days), and expensive. Enzyme treatments may also be augmented with caustic treatments to enhance the removal of interferences (Löder et al. 2017). Increased steps and handling of samples also enhance the potential for introduction of contaminants, loss of targeted plastics, or alteration of their physical characteristics.
2.4.3 Physical Separation of Plastics from the Matrix: Filtration and Sieving
To permit more facile detection, plastics are typically retrieved from air, aqueous, or solid phases and concentrated on surfaces, e.g., filters or sieves. In some cases, particulates may then be transferred from the initial filter to a second for optimal spectroscopic analysis. Analyst goals must guide desired filter characteristics: plastic size retention, filter composition and thickness/structure, and compatibility with the chemical agents used and with the instrumental detection approach to be employed. Filter configuration (e.g., punched holes, fiber weaves, or sintered metal disks) may control particle size/shape retention and the ease of retrieval of retained plastics. As this step can be labor-intensive and result in microplastic loss, refinements are beneficial. In this context, Nakajima et al. (2019a) developed a stainless-steel sieve (32 μm mesh) apparatus resistant to common oxidizing agents but compact enough to submerge in a glass beaker. They reported its use reduced the number of collections, rinsing, and transfer steps and generated better microplastic recoveries than widely used filter-based methods.
Researchers should ensure filter constituent materials do not contribute plastics or interfere with spectroscopic detection of targeted polymers. Filters/sieves themselves can be constructed of a range of materials, including quartz, stainless steel, nylon, cellulose, silicon, silver membrane, gold-coated PC, alumina-based membrane, and TeflonTM (Löder et al. 2015; Oßmann et al. 2017; Wolff et al. 2019; Wright et al. 2019; Käppler et al. 2015). Wright et al. (2019) collected inhalable microplastics of several polymer types on a variety of filter types (quartz, polytetrafluoroethylene, alumina, cellulose, and silver membrane) and then evaluated their compatibility with Raman imaging. Best results were obtained with silver membrane filters. Käppler et al. (2015) investigated filter materials for FTIR and recommended a silicon-based membrane. Particulates can become entrained in fibrous surfaces such as quartz fibers (Wright et al. 2019), and some materials may release fragments or interfere with the spectra of the targeted polymers. Consequences will vary depending on the detection scheme applied (e.g., visible light, FTIR, or Raman) and the polymer types targeted.
2.4.4 Density and Other Physical Separation
Flotation of plastics and sedimentation of dense inorganic particulates (ranging from 1.6 to >2.4 g cm−3) is a common purification step, especially for sediments, sludges, and water samples. While some common polymers (e.g., PE and PP) exhibit densities lower than water, others are near neutral buoyancy or denser (PVC, PC, PS, PET, PA, ABS, tire rubber: 1.0–1.4 g cm−3; Teflon: 2.2 g cm−3). Note: the presence of intact air pockets in foamed polymers (e.g., PS or PU) will increase buoyancy. Water surface tension can keep even dense plastics at the surface for extended periods. The presence of polymer fillers and additives can also alter plastic behavior. For example, the densities of carbon black, calcium carbonate (CaCO3), and glass fiber fillers typically exceed 2 g cm−3. To achieve flotation of a range of polymer types, analysts have typically used concentrated saline solutions consisting of NaCl, NaI, sodium polytungstate, ZnCl2, and ZnBr2 (GESAMP 2019). Choices are based on effectiveness, cost, and safety. The behavior of the microplastics in said solutions should be carefully monitored. For example, Rodrigues et al. (2019) observed that salt can deposit on the surface of microplastics, increasing their overall density, leading to their sinking and loss.
A variety of devices have been used for the gravity separation of plastics from denser particulates. These include simple glass funnels (Rodrigues et al. 2019) to the elaborate stainless-steel Munich Plastic Sediment Separator (MPSS; Imhof et al. 2012). Retention of plastics in the settled solids, as well on the container sides, may decrease their recovery. Again, the presence of polymeric material in the construction of settling apparatuses could lead to possible sample contamination. Nakajima et al. (2019b) engineered a simple all-glass separator, the JAMSTEC microplastic-sediment separator (JAMSS unit). It is comprised of two glass plates (Fig. 2.3), the upper consisting of an open cylinder and the lower, a chamber with capacities of 30, 60, or 100 ml. The design is based on the Combined Plate or Utermöhl Chamber, long-used for examining settled phytoplankton. The approach is to settle the solids into the lower container and then to isolate these from the overlying water column (containing the more buoyant microplastics) by sliding the upper plate. The plastics can then be poured onto a filter or sieve, followed by water washes of the chamber to dislodge any adhering microplastics.
JAMSTEC microplastic-sediment separator (JAMSS) unit. (a) The upper plate (left) incorporates an open glass tube, while the lower plate (right) includes a cylindrical glass container. (b) Small, middle, and large models of assembled JAMSS, consisting of a cylindrical container of 30, 60, and 100 ml volume, respectively. (c) JAMSS during density flotation with sediment in the lower container. (d) Separation of sediment and supernatant by sliding the two plates against each other. (e) JAMSS can be placed on a magnetic stirrer to ensure the sediments are well mixed during microplastic flotation. (f) Microplastics in the supernatant in the upper tube are poured out and rinsed from the internal walls of the tube. (From Nakajima et al. (2019b). http://creativecommons.org/licenses/by/4.0/. doi: 10.7717/peerj.7915/fig-1)
Wang et al. (2018) evaluated the recovery of polystyrene nano- and microplastics from sewage sludge and soil . They noted that 100 μm microbeads were effectively recovered by ZnCl2 solution-based flotation, but smaller beads were not. They also evaluated flotation efficiency as a function of time, indicating substantial periods were needed to reach 90% for particles <5 μm. Möller et al. (2020) reviewed several extraction methods for removing plastics from soils, including electrostatic separation for dry solids, oil extraction (utilizing the lipophilicity of the plastics), froth flotation (using a stream of air), various density-based approaches, and magnetic separation using lipophilic nanoparticles functionalized with iron.
2.4.5 Solvent Extraction
If research goals do not require the visualizing and counting/characterization of individual particles, but rather quantifying the total mass of plastic in a sample, solvent dissolution/extraction of plastics from a sample matrix may be appropriate. Polymer solubility must be initially established. Solvents that are most effective for a given polymer typically have similar solubility parameters, and solvation typically increases with temperature (Miller-Chou and Koenig 2003). Separation of undesired co-extractives is typically necessary. As an example, Ceccarini et al. (2018) extracted dried sand collected along a beach transect by first refluxing at ~37 oC with dichloromethane (DCM). This was followed by a second extraction of the sand in the same device with xylenes at 135–140 oC to obtain remaining, less-degraded polyolefins. Molecular size distributions of the extracted polymers were determined by gel permeation (also known as size exclusion (SEC)) liquid chromatography (GPC). 1H NMR, FTIR, and Py-GC/MS analyses of extracts were also performed. It is noteworthy that the authors observed greater amounts of DCM extractable residues (presumably degraded polymerics) in the more UV-exposed dune and backshore sands than in the foreshore sediments, highlighting the role of polymer weathering.
In another example, Fuller and Gautam (2016) pre-extracted municipal waste and soil samples in a pressurized fluid solvent extractor (Dionex ASE-350). Between 2 and 10 g of dried sample were extracted. They first were extracted with methanol at 100 °C to remove soluble fats and oils. The solids were then re-extracted with DCM at 180 °C. The solvent was removed by evaporation, yielding a solid residue, wherein the various polymers from the original sample were intermixed. FTIR analysis indicated spatial homogeneity in the resulting solidified plastic . The resulting FTIR spectra may be complex, as they will represent a composite of the different polymers present. If the extract is sufficiently free of nonplastic co-extractives, gravimetric determination of polymer mass allows a concentration determination. The authors (Fuller and Gautam 2016) performed recovery studies with PE, PP, PVC, PS, and PET. They suggested the method could be applicable to PU and PC, based on their detection in field-collected samples. Advantages include the simplification of cleanup/isolation procedures and the ability to automate analysis and quantitate total plastics, regardless of particle size. Again, most spectroscopy-based microscopic approaches focus on individual particle counts and are either instrument, time-intensive, or ineffective for microplastics below about 20 μm (Wolff et al. 2019). While data on microplastic shape and size were not obtained by Fuller and Gautam (2016), pre-separation of solids by passage through a sequence of different sized sieves could yield insightful data on particle size characteristics. The simplicity and ease in automation of the extraction method would provide some relief from the increased sample numbers.
2.5 Microplastic Detection and Instrumentation
Synthetic polymers are complex, typically high molecular weight organic molecules, and thus share attributes with natural dissolved (DOM) and particulate organic matter (POM). Hence, consideration of analytical techniques useful in studies of those materials may be fruitful (e.g., Materić et al. 2020). Being commercial products, extensive analytical work has also been done on polymers for developmental and quality control purposes and may also serve as a rich source of techniques. Indeed, considerable literature from the industrial plastic perspective has been available for years (e.g., Hakkarainen 2012). When selecting a mode of detection, the range of analytes (e.g., polymer types and sizes) to be included and particle characteristics (weight-based concentrations, particle abundances, or shapes) to be measured must be considered. In theory, it is desirable to obtain as complete as possible a suite of plastic particle characteristics. However, this is rarely achieved, even when using highly sophisticated instrumentation, due to the diversity of microplastic morphologies and polymeric compositions. Tradeoffs between detailed characterization of a limited number of particles and large sample throughput must be made. Complex analytical schemes (due to financial, temporal, and manpower resource limitations) restrict the number of samples that can be examined. Several detection techniques described here are themselves hybrid approaches, i.e., consisting of an initial analyte introduction or separation process (e.g., chromatography, thermal desorption, pyrolysis), followed by compositional measurement proper (e.g., MS, FTIR, or Raman). Each technique should be selected based on its ability to answer the desired research questions. A table detailing discussed techniques, as well as their limitations in addressing different research questions (Table 2.1), is presented.
2.5.1 Visual Identification
Many investigations of plastics in the environment rely on the initial visual identification of particles using light microscopy, e.g., dissecting scopes. Such equipment is widely available. In the course of such studies, relatively large plastic debris may be removed with forceps for additional evaluation. Plastic debris identification criteria used by human observers include shape, color, texture, and absence of internal structures. However, such decisions are vulnerable to error depending on observer experience, matrix, and particle characteristics. Visual assessment of melting characteristics may be useful by contacting the particle with a hot needle (discussed later). Reliability of identification drops with decreasing target size but smaller particles will typically outnumber larger ones. Microplastics often are homogeneous in hue (although differential weathering may alter this) and lack internal structures or organelles. Filamentary structures are common in nature, so fiber identification imparts additional concerns. Fibers present narrow cross-sectional areas for examination and thus may be misidentified. Transparent particles may be overlooked using light microscopy. An interesting advanced technique is the use of “optical tweezers” for shepherding nanoplastics in liquids. Gillibert et al. (2019) demonstrated this, in combination with Raman microscopy, on a range of micro- and nanoplastics in fresh and saltwater, as well as particles that exhibited a thin biofilm .
2.5.2 Dyes and Fluorescence Microscopy
A number of stains have been evaluated for visualizing microplastics. Lipophilic Nile red has become a popular choice (Maes et al. 2017). This dye fluoresces, facilitating the detection and counting of small particles (Erni-Cassola et al. 2017). Prior digestion of coincident natural organic matter (e.g., cellulose and chitin) in the sample is recommended, as these polymers may also absorb dye to varying degrees. PE, PP, PS, and PA absorb the Nile red and fluoresce intensely; less hydrophobic polymers (e.g., PC, PET, PVC, and PU) absorb less and exhibit less intensity (Erni-Cassola et al. 2017). As brightness and particle size affect detectability, smaller particles and less lipophilic polymer types are more difficult to quantify by this approach due to their fainter signals. The fluorescent dye technique facilitates the identification of individual particles and shapes but is less diagnostic for polymer type. Image analysis software allows calculation of approximate plastic mass, based on the particle area and assumed density. As polymer thickness and composition (and hence density) are unknown, this approach can encompass considerable error. Flow cytometry, widely used for cell counting in phytoplankton and hematology research, has occasionally been used in microplastic-related lab experiments (e.g., Summers et al. 2018; Woods et al. 2018; Fu et al. 2020) but less so in field monitoring efforts. In this technique, particles in suspension are focused “single file” with a sheath fluid and passed by a laser. Impinging light may then be scattered forward or sideways, as a function of the size and granularity of the particle, respectively. Additionally, absorption may occur and particle fluorescence measured. Treatment of microplastics with Nile red might be advantageous here. Prior digestion of samples with chemical agents to remove biofilms or oxidize biogenic particulates might also facilitate detection. A major limitation of using dyes and fluorescence microscopy, however, is that the dye may interfere with subsequent polymer identification. Further, it has been shown that fluorescent compounds may leach from the polymer in tissues, so fluorescence itself may not be a reliable indicator of particle location (Schür et al. 2019).
It should be noted that fluorescent pigments, dyes, and optical brightening or whitening agents are widely used in the plastic and textile industries (Christie 1994). Thus, certain types of plastics may be detected without the addition of dyes. Dehghani et al. (2017) employed fluorescence microscopy to assess microplastics in urban dust samples. Bulk street sweeping samples were collected from the central district of Tehran, Iran. Fluorescent particles and fibers were visible in all samples. Hale et al. (2020) observed that colored PU foam commonly used in gymnastic pits fluoresced strongly. Additionally, <53 μm microplastics (produced by cryogenic fragmentation of bulk foam) were readily ingested by brine shrimp larvae in the lab. These microplastics were easily observed within the digestive tract by fluorescence microscopy (Fig. 2.4).
Image of polyurethane microplastics (<53 μm) ingested by brine shrimp nauplii (Artemia sp.). Additives within the polyurethane elicited a fluorescent response. Imaged on an Olympus
FV1200 laser scanning confocal microscope. Credit: Hamish Small (VIMS) and Virginia Worrell (Virginia Governor’s School)
2.5.3 Electron Microscopy (EM)
While not typically used for direct polymer identification, scanning electron microscopy (SEM) is a powerful technique for delineating minute structural features, including deformities in, as well as colonizing organisms on, plastic debris surfaces (Zettler et al. 2013; Gniadek and Dąbrowska 2019). EM exhibits orders of magnitude greater spatial resolution (~0.0004 μm) than light microscopy (~0.3 μm). This derives from the differences in wavelengths between visible light (400–700 nm) and the high-energy electrons (0.001–0.01 nm) used for illumination (Girão et al. 2017). This concept also contributes to the resolution limitations of IR spectroscopy (2.5–20 μm). High-energy electron beams in EM are, however, capable of altering/damaging specimens, so care must be taken when imaging. Instrumentation and sample preparation costs for EM are also much greater than for light microscopy. The technique is not suitable for inspecting large microscopic fields or for rapid sample throughput. EM can be combined with energy-dispersive X-ray spectroscopy (EDS) to provide additional elemental composition information of targets (Girão et al. 2017). For example, Wang et al. (2017) applied SEM/EDS to evaluate elemental signals from selected particles. They succeeded in identifying the presence of chlorine in PVC microplastics, as well as ruling out nonplastic minerals in samples. Fluorinated polymers may also be amenable to this technique. Fries et al. (2013) identified the presence of Ba, S, O, and Zn associated with Wadden Sea microplastics with SEM/EDS. They also detected TiO2 nanoparticles, which they theorized were used as white pigments or UV blockers in plastics. Ghosal and Wagner (2013) used SEM/EDS to identify Br and Sb, components of polymer organic and inorganic flame retardant additives, as well as TiO2 in residential dusts containing microplastics. Further, they applied micro-Raman spectroscopy (μRaman) to associated particles and identified PE. They suggested ingestion of such microplastics could be an important human exposure pathway for these additives. Care must be exercised to remove possible matrix interferences from samples. For example, Br and Cl ions are common in seawater and residues may complicate conclusions.
2.5.4 Chromatography
Chromatography is widely used to separate components of complex mixtures by their properties, including polarity, solubility, volatility, and molecular size. Systems typically consist of a sample inlet, chromatographic column, and a detector. Analytes chemically or physically interact with a stationary phase contained within the column and are transported through the column by a mobile phase (e.g., a gas, liquid, or supercritical fluid). Analyte retention time (or volume) and detector response (e.g., a characteristic spectrum) are used for identification. Gas chromatography (GC) requires volatilization of analytes and employs a gaseous mobile phase. As such, GC has limited applicability to the direct analysis of low volatility polymers. However, it is invaluable for separating volatile constituents (e.g., additives), as well as polymer thermal degradates (see section on pyrolysis). Its facile coupling with low-cost mass spectrometers greatly expands its value.
Liquid chromatography (LC) in the form of size exclusion liquid chromatography (SEC), also known as gel permeation chromatography (GPC), has considerable utility in polymer analysis. Analyte elution time is a function of the pore/exclusion size of the media. Traditionally, larger molecules emerge earlier from the column than smaller molecules (which take a more circuitous path through the media). Hence, it has efficacy for both purification and characterization of polymeric materials. SEC has been widely used in polymer science, engineering, and product quality control, less so, to date, in the delineation of plastic contamination of the environment. Advancement in MS interfaces capable of handling liquid eluents has increased the power of this technique. LC has also been coupled to a variety of spectroscopic detectors, and their use is now appearing in the plastic debris-related literature. For example, Hintersteiner et al. (2015) evaluated molecular weight distributions of olefinic microplastics, following isolation from the sample matrix (in this case, personal care products). They dissolved the microplastics in 1,2,4-trichlorobenzene at 160 oC and separated the polymers by molecular size using high-temperature SEC with IR spectroscopy detection. Calibration was accomplished using PS standards ranging from 700 to 2 million g mol−1. LC typically exhibits lower resolution capabilities than GC. However, the development of ultrahigh performance (UHP) LC instruments, equipped with columns containing particles of extremely small size, has reduced this disadvantage.
Supercritical fluid chromatography (SFC) often exhibits higher-resolution capabilities than LC and has been used to characterize polymers (e.g., Takahashi 2013). In SFC the pressure and temperature of a gas (e.g., CO2) are manipulated in such a way that it exhibits properties intermediate to a gas and liquid. Commercially available SFC equipment has become widely available, as are improved interfaces compatible with modern detectors. However, no published references were found to indicate it has yet been used for the analysis of microplastics obtained from environmental media.
2.5.5 Infrared (IR) Spectroscopy
IR spectroscopy, commonly performed with a Fourier transform infrared (FTIR) spectrometer, is an established technique for identification of polymeric materials. IR spectroscopy is a nondestructive technique, allowing reanalysis of the same material. The two primary modes most commonly applied for microplastic analysis are transmittance and reflectance (Chen et al. 2020). The long wavelengths of IR radiation limit the spatial resolution of FTIR to 2.7 μm, but instrument limitations reduce the spatial resolution to about 10–20 μm, depending on the design. Compositional identification is achieved by comparing the sample spectrum with a known reference polymer. FTIR may be suitable for the identification of colored microplastics whose pigments may fluorescence and interfere with Raman spectra. FTIR may also be valuable for monitoring the degree of weathering of polymers, e.g., the development of hydroxyl and carbonyl groups (Cai et al. 2018).
For larger plastic debris (>500 μm), FTIR can be paired with an attenuated total reflectance (ATR) accessory, which measures the surface composition of materials. The IR from an ATR typically penetrates the polymer to a depth of 0.5–2 μm (Li et al. 2018b), problematic for heterogeneous, layered materials. The measurement requires physical contact of a crystal (composed of germanium, zinc selenide, silicon, or diamond) with the targeted material. This requirement can lead to logistical issues with some materials, e.g., small micro- or nanoplastics, and is not amenable to samples with numerous targets. However, ATR-FTIR has been used to identify larger microplastics in Arctic deep-sea sediments (Bergmann et al. 2017) and in fish from the African Great Lakes (Biginagwa et al. 2016).
Micro-FTIR (μFTIR) combines vibrational spectroscopy and microscopy, allowing the analysis of smaller particles. Use in transmission or reflectance mode permits application using membrane filters with minimal sample preparation. Transmission mode requires an IR-translucent substrate, while the reflectance mode can be applied to thick and more opaque samples (Li et al. 2018b). Application to irregularly shaped particles can result in non-interpretable spectra, due to refractive errors (Harrison et al. 2012). Li et al. (2018a) used μFTIR to demonstrate that supermarket-purchased mussels in the UK contained microplastics and concluded that their quantification should be included in food safety management measures.
Manual repositioning of a μFTIR stage across a viewing field containing numerous particles is subject to human bias, tedious, and time-consuming. Field-derived samples may contain hundreds of candidate particles. As a consequence, commercial instruments have been developed to automate this process and generate spectral image maps. However, the process can still be slow (hours to days), as a function of required resolution and sample complexity. That being said, μFTIR analysis is typically faster than μRaman (described below). Instrument cost increases with increased capabilities and in the present market may be beyond many lab budgets.
The use of focal plane array (FPA) μFTIR detectors allows for analysis of multiple particles at one time with high resolution. Such FTIR imaging can produce a detailed, high-throughput analysis of total microplastics on a filter. However, particles with irregular shapes may still not be suitable for FPA-μFTIR imaging. Additionally, only a few μFTIR instruments can analyze particles <20 μm. Fibers, due to their narrow cross-sectional areas, also present challenges. However, Tagg et al. (2015) successfully demonstrated that FPA-based μFTIR imaging could identify a range of microplastics in wastewater. Primpke et al. (2017) developed an automated image analysis method using FPA-μFTIR to provide particle identity, count, and size of microplastics in complex matrices, increasing data quality and ease of data interpretation. Later, Primpke et al. (2018) generated a FTIR reference library for automated analysis of microplastics. Other reference libraries and automated sampling software are available, such as siMPle (simple-plastics.eu; Liu et al. 2019; Primpke et al. 2019).
2.5.6 Raman Spectroscopy
Akin to FTIR, Raman is a nondestructive, vibrational spectroscopic technique increasingly used in the analysis of plastic debris. Unlike IR spectroscopy, where the absorbance of radiation by molecules is measured, in Raman a narrow wavelength laser is used to excite the surface of a particle, and the photons resulting from inelastic scattering (Raman scattering) are detected and recorded as a spectrum. The spectrometer may be interfaced with a microscope (designated μRaman). Compared to FTIR, Raman is adept at identifying nonpolar functional groups (e.g., aromatic, C—H, etc.; Käppler et al. 2016). Hence, IR spectroscopy and Raman are complementary techniques. μRaman typically has greater spatial resolution potential than μFTIR, down to about 1 μm. However, fluorescence from irradiated materials can overwhelm the relatively weak Raman scattering. But the analyst may be able to minimize fluorescence by choosing a different excitation wavelength. Both FTIR and Raman are typically used to analyze particles on a filter or other surface, following pretreatment of sample to eliminate interfering materials. The filter or window on which the samples are placed may limit the wavenumber range possible in transmission mode.
Raman can identify plastics from a variety of environmental matrices. However, to date available studies are heavily skewed towards water and sediments (most recently reviewed in Erni-Cassola et al. 2019). Recently, Wright et al. (2019) applied μRaman spectral imaging to the identification of microplastics in inhalable air, while simultaneously evaluating routine air quality monitoring metrics. Fortin et al. (2019) characterized microplastics <10 μm by μRaman in water from an advanced wastewater treatment facility. Cabernard et al. (2018) reported that μRaman identified more particles in the 10 to 500 μm range. Particle agglomeration and losses were observed, suggesting the need for surrogate spiking and percent recovery studies in the microplastic analysis.
Researchers are developing open-access reference libraries for Raman spectra. For example, Munno et al. (2020) established a spectral library of plastic particles (SLoPP) encompassing 148 diverse reference spectra. Their SLoPP-Environmental or SLoPP-E libraries included spectra of 113 particles collected globally. This addition is important as weathering can modify spectral characteristics. When compared to manufacturer reference libraries, 63% of particles tested registered the strongest matches using SLoPP or SLoPP-E, illustrating the utility of reference libraries created specifically with microplastic identification in mind.
Two notable studies have compared μRaman to FPA-μFTIR for analysis of microplastics in environmental samples. Käppler et al. (2016) found that Raman was superior at identifying PVC particles, while polyesters and particles with high dye content or fluorescence were more accurately identified with FPA-μFTIR. Due to this and its greater resolution, μRaman detected significantly more particles than FPA-μFTIR. Yet, when methods were automated to reduce the analysis time from 38 h to 90 min (closer to the 20-min sampling time of FPA-μFTIR), the same number of particles was identified. This underscored the opinion that longer processing times are necessary to reap the advantages of Raman. Cabernard et al. (2018), in a study of North Sea surface waters, arrived at similar conclusions. These authors reported that the Raman signal was obstructed by highly pigmented particles, limiting identification, particularly for rubber and ethylene vinyl acetate (EVA) microplastics. Käppler et al. (2016) identified TiO2 in inorganic particles by Raman. The TiO2 peak was also identified as a white pigment, along with an acrylic resin using FTIR. Imhoff et al. (2016) also identified paint particles using Raman by their characteristic high pigment content.
2.5.7 Scanning Probe Microscopy (SPM)
SPM is a family of technologies that has been widely used in materials science. Atomic force microscopy (AFM) uses a mechanical cantilever to physically measure the surface topography of a sample at low micron and even sub-nanometer scales (Dazzi et al. 2012). The interaction volume between the physical probe and sample may be on the picometer scale.
The high resolution of AFM can be invaluable for characterizing minute physical features, as small as an atom. However, instruments can typically only interrogate micron-sized spatial areas and must be used in workspaces carefully engineered to eliminate ambient vibrations. In AFM-IR, the thermal expansion of materials following the incidence of IR radiation is measured (Fu et al. 2020).
Optical photothermal IR (O-PTIR) is a novel technique that uses a visible laser light source, rather than a mechanical cantilever, to evaluate thermal expansion of the targeted material following illumination with a collinear, tunable mid-IR laser (Fig. 2.5). This approach allows the extraction of a signal that closely approximates widely available FTIR spectra. Spatial resolution is <1 μm, far lower than conventional IR. Since the system is not based on IR transmittance, thicker samples can be evaluated than with conventional IR absorption. It is also compatible with irregular surfaces. The system can be coupled with Raman spectroscopy, allowing the collection of Raman and IR spectroscopy data for the same particle. As these techniques are complementary, identification capabilities are increased.
O-PTIR employs a visible light (in this case, 532 nm) detection laser to evaluate target absorption of IR from a second tunable laser. As such, the approach is not subject to typical IR refraction limitations, permitting sub-micron spatial resolution. The system here was combined with a Raman spectrometer, allowing the collection of complementary spectral information. IR and Raman spectra were obtained from several locations (shown as green, blue, and purple colors) across a 4 × 14 μm microplastic particle present in NIST SRM#2585 (indoor dust). Spectra were consistent with a polymethacrylate polymer. (Images courtesy of Photothermal Spectroscopy Corp)
Merzel et al. (2019) recently applied AFM-IR, O-PTIR, and fluorescence microscopy to image and chemically interrogate nanometer-sized PS beads taken up and retained by freshwater mussels during an in-laboratory exposure . They noted, while sensitive, AFM-IR analysis was time-consuming and applicable only to the immediate surface of the material examined. The presence of a surface biofilm also interfered with spectra acquisition for the underlying polymer. In contrast, O-PTIR was faster and less vulnerable to biofilm interference.
2.5.8 Mass Spectrometry (MS)
MS is a powerful tool for the identification and quantification of organic materials. The technique measures the mass-to-charge ratio (m/z) of ions generated from the fragmentation of an analyte. A variety of ionization approaches, differing in energy, have been developed, depending on the material under study. MS with specialized interfaces can be utilized for the identification of polymers, degradation products, and additives. Interfaces include chromatographic (i.e., GC and LC) and thermal techniques (i.e., pyrolysis (Py), thermogravimetric analysis (TGA), thermal extraction desorption (TED)) for better characterization of different type of plastics. Spectra acquisition is very rapid, on the order of milliseconds, depending on the instrument design and desired mass range. MS is popular to identify and quantify both targeted and untargeted additives (e.g., plasticizers (Peters et al. 2018), flame retardants (Hale et al. 2002; Khaled et al. 2018), and pigments (Imhof et al. 2016)) in plastic debris, as well as surface-sorbed contaminants.
High-resolution and tandem (where two or more mass analyzers are coupled) MS instruments are becoming more widely available. However, MS (with the exception of pyrolysis applications) for the analysis of plastics present in environmental matrices has to date been limited. This is due to the relatively low mass range of most MS compared to that of many polymers. MS units capable of high molecular weight analyses tend to be expensive and complex and may require considerable operator skill.
Limited or no sample preparation is a desirable feature. In some cases, ambient (direct) analysis of plastics is feasible by probing surfaces with an energetic beam. However, these techniques may not be applicable to molecules with molecular weights >3000 Da (Schirinzi et al. 2019). In other approaches polymers are dissolved, separated from the matrix (via vaporization, pyrolysis, or liquid chromatography), and then detected by MS.
Schirinzi et al. (2019) evaluated several MS-based techniques for the analysis of PS microplastics obtained from natural waters, including SEC/atmospheric pressure photoionization (APPI) MS, direct analysis in real-time (DART), matrix-assisted laser desorption ionization (MALDI), and desorption electrospray ionization (DESI). They reported that SEC/APPI-MS exhibited the greatest sensitivity, with a detection limit of about 20 pg. MALDI-MS has been explored for the detection of various synthetic polymers (Weidner and Trimpin 2011). A challenging aspect of MALDI-MS analysis is the identification and discrimination of plastic fragments in the presence of coincident interferences, e.g., biofilms. Lin et al. (2020) recently used MALDI-MS to identify and quantify PS particles ranging from 100 nm to 4 mm. Signals were enhanced by thermal pretreatment, enabling higher quantification accuracy. Lin et al. (2020) examined the feasibility of such methods to analyze commercial plastic products, as well as microplastics from river water and fish. MALDI-MS thus is a promising tool for the evaluation of limited numbers of microplastics in samples (Huppertsberg and Knepper 2018).
In a novel study, Wang et al. (2017) depolymerized ester-containing polymers (i.e., PC, PET) using alkali and heat. They then subjected the resulting products (bisphenol A and p-phthalic acid) to liquid chromatography/tandem mass spectrometry (LC-MS/MS). Sample matrices included wastewater sludge, marine sediments, indoor dust, and shellfish. As contributions of bisphenol A and p-phthalic acid from non-polymer sources were possible, samples were also assessed for their pre-depolymerization levels to differentiate pre-existing contamination by these constituents. The authors reported particularly high concentrations of PC (246 mg/kg) and PET (430 mg/kg) in the dust sample.
Materić et al. (2020) applied thermal desorption-proton transfer reaction-mass spectrometry (TD-PTR-MS) to detect nanoplastics of several polymer types in 0.2 μm-filtered water samples derived from snow cores from the Austrian Alps. TD-PTR-MS has mainly been employed for volatile compounds but has recently been extended to semivolatiles. Materić et al. (2020) used chemical ionization via hydronium ions to produce low fragmentation ions. Evaporation/sublimation of constituents was achieved by ramping from 35 to 350 oC at 40o/min.
Time-of-flight secondary ion mass spectrometry (TOF-SIMS) is capable of providing compositional and spatial distribution information for plastics. Excitation of a surface by a focused ion beam causes an emission of secondary ions and clusters from the sample. The TOF analyzer then measures the exact mass of these, allowing for compositional determination. The resulting data can be used to create images of very thin polymer surfaces (on the order of nm). For example, Jungnickel et al. (2016) applied this technique to image 10 μm PE particles.
Direct analysis in real time (DART) is an ion source that uses a heated helium, argon, or nitrogen plasma stream to desorb and excite molecules from surfaces. These may then be drawn into the inlet of a high-resolution MS, permitting exact mass measurements and subsequent constituent identification. Zhang et al. (2020b) introduced selected microplastics into a thermal desorption/pyrolysis inlet connected to a DART ionization device interfaced with a Q ExactiveTM hybrid quadrupole-orbitrap MS. They reported the detection of both additives (plasticizers, antioxidants, and cross-linking agents) and polymers (PE, PP, PET, PS, polyester, PA). Multivariate statistical evaluations of the ions produced from the thermal desorption and pyrolysis processes were used to establish identifications.
Elemental analyzer/isotope ratio mass spectrometry (EA/IRMS) is widely used in geochemistry to establish the origin of organic matter using carbon isotopes and to evaluate food authenticity (e.g., via nitrogen isotope patterns). Berto et al. (2017) demonstrated that carbon isotopic composition is sufficient to discriminate fossil fuel-derived polymers (e.g., high- and low-density PE) from plant-derived bioplastics in commercial products. This method also was advantageous for testing darkly colored samples, which are problematic in some spectroscopic techniques. However, EA/IRMS alone provides limited information about the specific type, shape, size, and composition of MPs.
Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS) has long been used for the characterization of synthetic polymers (e.g., Brenna and Creasy 1991), petroleum residues (Chen et al. 2016), and natural organic matter (Riedel and Dittmar 2014). FTICR-MS is a promising technique, allowing the accurate mass analysis of high molecular weight species. However, it has not been widely applied to the issue of plastic debris to date. Instruments are costly.
Inductively coupled plasma MS (ICP-MS) has occasionally been employed to characterize a range of elements, particularly metals (common in pigments) associated with plastics. Samples are typically digested using strong acids prior to analysis. See the pigment discussion below for more details.
2.5.9 Thermal Analysis Techniques
A variety of thermal analysis techniques are applicable to microplastics. A simple approach is the application of a hot needle to selected particles to evaluate melting potential (Silva et al. 2018). Natural materials do not typically melt or curl. Peñalver et al. (2020) recently reviewed a number of thermal analysis techniques. These range from gravimetric measurements across time and temperature gradients, to more sophisticated hyphenated techniques. Gravimetric measurements, being rather nonspecific, are vulnerable to the presence of interferences from nonplastic materials. Detection limits may be problematic as many approaches (e.g., pyrolysis) are sample size limited. Pre-concentration of microplastics may reduce this shortcoming.
Pyrolysis-GC/MS (Py-GC/MS) has proven to be a valuable technique for microplastic characterization. This destructive method thermally deconstructs samples over one or multiple temperature ranges. Polymers that produce characteristic degradates may thus be identified and quantitated. Further, GC peaks for additives or sorbed hydrophobic organic pollutants may be identified using an additional thermal desorption step. Py-GC/MS has typically been employed for discrete plastic particles. More recently, it has been adapted to analyze mixtures, allowing higher-throughput analysis. As it is not dependent on particle size, Py-GC/MS is a promising technique for nanoplastic analysis (Mintenig et al. 2018; Ter Halle et al. 2017), provided sufficient material is input. Unlike FTIR or Raman methods, however, pyrolysis by itself does not permit the acquisition of particle counts, shape, or size data, unless other techniques (or pre-sieving into size classes) are employed prior to pyrolysis.
A sample is typically introduced to the pyrolyzer in a glass thermal desorption tube or stainless-steel cup. These can accommodate small pieces of plastic, generally less than 1.5 mm diameter. The development of larger pyrolysis chambers would advance this technique, allowing characterization of microplastics that have been concentrated on a filter. For polymer analysis, high temperatures (~700 °C) are required. At lower temperatures (590 °C), pyrolysis is possible with thermochemolysis, established by spiking the sample with, for example, ~10 μl of tetramethylammonium hydroxide (Gomiero et al. 2019; Fischer and Scholz-Böttcher 2017). Pyrolysis thermally degrades the polymer, yielding organic pyrolysates, which are characterized via subsequent GC/MS. Comparison of the chromatographic fingerprint to that of known polymers can reveal polymer type and peak integration of one or more marker compounds used to obtain analyte mass (Fischer and Scholz-Böttcher 2017). For example, in a study of plastic pollution analysis using Py-GC/MS, Fries et al. (2013) presented a PE chromatogram containing a characteristic series of n-alkanes, n-alkenes, and n-alkadienes (also shown in Ter Halle et al. 2017). A comprehensive list of characteristic decomposition products for different polymers can be found in Fischer and Scholz-Böttcher (2017). Fries et al. (2013) were able to identify eight polymer types collected from sediments using this technique, including PE, PP, PS, and PA. Likewise, Doyen et al. (2019) and Gomiero et al. (2019) identified multiple polymers in plastics extracted from beach sediments, while Hendrickson et al. (2018) used Py-GC/MS to validate polymer identification in surface waters of Lake Superior. Py-GC/MS has the ability to characterize the relative contribution of different polymers in complex, layered polymers, or copolymers.
Complex samples with multiple unknown polymers may be analyzed using Py-GC/MS. For example, Ter Halle et al. (2017) used ultrafiltration to concentrate nanoplastics in seawater and then validated their presence using dynamic light scattering analysis. The thermal degradation products were compared to known polymer standards, and advanced statistics were applied to estimate the relative percent contribution of up to three polymers per sample. Weight-based estimates were not possible using this approach. An alternative statistical approach can be found in Zhang et al. (2020b). The complexity of these analyses was underscored by the shared pyrolysis products from multiple compounds (Fischer and Scholz-Böttcher 2017). Further, coincident matrices in the sample can contribute thermal decomposition products, such as styrene from chitin (Fischer and Scholz-Böttcher 2017). Sample digestive pretreatment may be effective in eliminating much of this interference.
An additional capability of Py-GC/MS is investigating polymer additives or particle-sorbed contaminants. To detect volatile or semi-volatile organic additives, thermal desorption is typically employed prior to pyrolysis. Here, a sample is first heated to 350 °C, for example, in order to release the more volatile constituents. Cryogenic cooling is employed to trap thermal decomposition products prior to GC and subsequent pyrolysis of the sample. Fries et al. (2013) used this “double-shot” technique to detect phthalates, benzaldehyde (flavoring substance), and 2,4-di-tert-butylphenol (antioxidant) in plastics. Alternatively, in place of separate thermal desorption and pyrolysis steps, compositional data may be collected over a temperature ramp whereby thermally desorbed compounds will elute early, while pyrolysis decomposition products elute later. Zhang et al. (2020a, b) demonstrated this approach, in conjunction with high-resolution MS. As such, Py-GC/MS can simultaneously investigate complex organic compounds within/on plastics not feasible using IR or Raman spectroscopic techniques.
A related technique to Py-GC/MS is thermal extraction desorption GC/MS (TED-GC/MS). This approach employs thermal decomposition over a longer period of time, utilizing a thermal desorption unit (Duemichen et al. 2019). This process is amenable to larger samples and reduces the need for pretreatment. Eisentraut et al. (2018) used TED-GC/MS for the analysis of street runoff and sludge samples. Street runoff was sieved and reconcentrated on glass fiber filters, while sludge was homogenized with a ball mill. In addition to traditionally identified thermoplastics (i.e., PE, PP, etc.), these authors reported the presence of tire wear particles using marker compounds (elastomers, antioxidants, and vulcanization agents). Overall, TED-GC/MS and Py-GC/MS hold promise for the characterization of plastic debris, independent of size, and for increasing sample throughput.
Thermogravimetric analysis (TGA) is a method where the loss in sample mass is evaluated as a function of temperature, under specific atmospheric conditions. Without additional detectors such as MS or IR, qualitative information provided is limited. Sample pretreatment is typically modest. The relatively high sample amounts employed in TGA units (about 200 times higher during TED-GC/MS than Py-GC/MS) enable the measurement of heterogeneous matrices, reducing sample representativeness concerns (Dümichen et al. 2015; Elert et al. 2017). However, further investigations of the applicability of this method for matrices with high concentrations of impurities, such as natural organic matter, are needed. Mengistu et al. (2019) utilized a simultaneous thermal analyzer, a TGA interfaced with a FTIR, to systematically evaluate responses from tire granules and sediments amended with tire granules. Here, both sample mass losses over a temperature and time program and IR spectral information were obtained. The formulated sediments (wetted to 50% by volume) consisted of 5% organic matter (conifer bark), 75% quartz sand, and 20% kaolinite clay.
2.5.10 X-ray Photoelectron Spectroscopy (XPS)
XPS, also known as electron spectroscopy for chemical analysis (ESCA), is a technique for qualitative and quantitative measurement of elemental composition and chemical /electronic state of elements in materials. X-ray irradiation of a surface produces spectra denoting spatial and depth distribution. The kinetic energy and number of electrons that escape from the surface (1–12 nm) of the material are simultaneously measured. Hernandez et al. (2017) used XPS to evaluate the chemical composition of PE nanoplastics in personal care products, while Lu et al. (2018) investigated the aqueous aggregation of PS microspheres under varying pH, humic acid, and ionic conditions.
XPS can also provide valuable information on chemical changes occurring in the first atomic layer (<3 nm) of polymeric surfaces. Tian et al. (2019) examined the formation of C–O groups on PS nanoplastics after 48 h of UV irradiation. In contrast, no significant changes were observed by FTIR analysis. Future application of XPS to the study of micro- or nanoplastic surface weathering under environmental conditions may be insightful. Furthermore, XPS may facilitate a deeper understanding of biofilm formation on plastics. In this context, Feng et al. (2018) explored changes via XPS in extracellular polymeric substances released by microorganisms in activated sludge.
2.6 Microparticle Classes of Emerging Concern
Many researchers exclude biogenically-derived or partially synthetic plastic debris (e.g., cellulose-derived products such as rayon) from their consideration of “microplastics” in the environment. However, organismal effects may occur via physical mechanisms, irrespective of the particle’s precursors (fossil fuel, bio-based, or wholly natural). For example, negative impacts may arise from physical (e.g., blocking of digestive tracts or overall reduction of caloric value of ingested materials) rather than chemical interactions (Wright et al. 2013). Suaria et al. (2020) reported that naturally occurring and man-made cellulosic fibers outweighed by >10-fold synthetic microfibers in the waters from six ocean basins. Reed et al. (2018) reported that rayon fibers were the most abundant microplastic class detected in marine sediments near the Rothera Research Facility (Antarctica). These likely arose from textiles released via wastewater. Further, biopolymers have been touted as a replacement for fossil fuel-based polymers. However, the mechanisms of toxicity of microparticles remain uncertain.
Analysis of natural precursor-derived particles (e.g., rayon) can be problematic due to their vulnerability to caustic sample preparation techniques commonly used for wholly synthetic microplastics. In addition, their chemical similarity to natural detritus may confound subsequent instrumental analysis. Surface coatings and tire wear are two additional classes of particles that are environmentally prevalent. However, to date, they have not garnered the level of attention paid to other microplastics. In part, this arises from their complex nature and difficulties in their analysis.
2.6.1 Surface Coatings/Paints
Buildings, roadway markings, and vessels are frequently treated or painted. Modern surface coatings often have polymeric components. Fragments are released as a result of abrasion and weathering. Paints may be formulated with pigments containing metals (e.g., Ba, Cd, Cr, Cu, Fe, Hg, Pb, Sn, Ti, and Zn) and organic constituents. Specialty coatings may contain intentionally toxic chemicals such as antifoulants and microbicides. Takahashi et al. (2012) observed that paint particles constituted up to 0.2% of cores of UK estuarine sediments. Here, particles were manually separated and weighed. The authors noted that metallic entities were rather homogeneously distributed vertically in the cores. They hypothesized the metals, being more water-soluble, may have been released from the pigment particles.
Song et al. (2014) investigated microplastics in the waters of Jinhae Bay, Korea. They observed that paint particle abundance exceeded that of other microplastic classes and that most paint particles were small, less than 100 μm. Alkyd resins and poly(acrylate/styrene) from fiberglass were common. Particles concentrated in the water’s surface microlayer, a zone of considerable physical and biological activity. Microplastics were collected on GF/F glass fiber filters and identified by ATR-FTIR. Imhof et al. (2016) investigated microplastics and paint particles in freshwater lake sediments. They reported that paint particles were small: 1–50 μm. Hence, they exhibited high surface area to volume ratios. Samples were treated with hydrogen peroxide and sulfuric acid to remove organic interferences. They were stirred for 1 week and collected on 2.2 μm quartz fiber filters (Whatman QM-A). Microplastics were identified by μRaman. Particle abundances were determined by counting selected regions of the filter. However, this approach may result in errors if the particles are heterogeneously distributed on the filter. The authors also noted that the pigment particles were brittle. Hence, rigorous physical treatment of samples could distort the original particle size distributions. In some cases, the authors were unable to identify associated polymers in the particles. Imhof et al. (2016) determined metal content by inductively coupled plasma MS. Cabernard et al. (2018) evaluated particles from North Sea surface waters by μRaman and ATR-FTIR and reported many large (>500 μm) “varnish” fragments (suggesting precursors derived from nonfossil fuel sources). Smaller particles were collected on a gold-coated mirror for μRaman or gold-coated PC filters for FTIR in reflectance mode. They also encountered colored particles that exhibited substantial fluorescence, interfering with Raman analysis. Aggregation of particles on the filter, reducing apparent particle numbers, was observed. The presence of dark pigments and fibers was an additional analytical challenge. Cabernard et al. (2018) also noted long analysis time, residual organics interfering with spectra, and the presence of salt precipitates from lab procedures compromised results.
2.6.2 Tire Particles
Tire wear fragments have been reported by some to be among the most abundant synthetic microparticles, especially near roadways (Kole et al. 2017; Sommer et al. 2018; Hale et al. 2020). In addition, substantial amounts of scrap tires are recycled, including use on playgrounds and athletic fields. In addition to the rubber infill, such fields may incorporate polymer fibers to simulate grass blades, as well as polymeric carpet backing (Cheng et al. 2014). Tire scrap and other waste plastics are also being incorporated into asphalt pavements. Materials are subsequently subjected to additional weathering and fragmentation to varying degrees. Fragments may be transported by runoff to surface waters. From there they may be transported by runoff to surface waters. Particle composition varies as a function of the manufacturer and may include natural rubber, carbon black, ABS plastic , metal and fiberglass belts, and other materials. A variety of techniques (Leads and Weinstein 2019) have been used to quantify tire wear, including visual examination, Py-GC/MS, SEM-EDX (scanning electron microscopy-energy-dispersive X-ray analysis), FTIR (for ABS), and investigation of specific chemical markers, such as benzothiazole.
Analysis of tire particles using spectroscopic methods (Sommer et al. 2018) can be impeded by near-complete absorption of IR light due to filler components in tire fragments (e.g., carbon black) and strong fluorescence (Eisentraut et al. 2018). Thermoanalytical methods, like Py-GC/MS and TED-GC/MS, are an alternative approach. These techniques utilize markers such as decomposition products and vulcanizing agents for identification. Eisentraut et al. (2018) used TED-GC/MS to simultaneously measure microplastics originating from thermoplastics and tire wear abrasion products in environmental matrices. Unice et al. (2012) developed a protocol to analyze and quantify tire particles in environmental matrices using Py-GC/MS.
Optimal markers should be selective, stable, and easily detectable. Benzothiazole-based vulcanization agents have previously been used as chemical markers to estimate presence (Spies et al. 1987). However, their suitability in quantification (and identification) of tire particles has been questioned due to their water solubility/leachability and reactivity (Eisentraut et al. 2018; Wagner et al. 2018; Klöckner et al. 2019). Zn as an elemental marker has potential due to its high concentration in tires. Unfortunately, there are numerous sources of Zn (Klöckner et al. 2019). Thus, using elemental markers like Zn for the detection of tire particles in environmental matrices may be suitable only if coincident interferences are removed prior to analysis of the fragments of interest.
Natural rubber (NR) is the main elastomeric constituent of truck tires but has been found in other tire materials (Wagner et al. 2018). NR decomposition products include dimers, trimers, and tetramers of isoprene. However, coincident natural sample constituents may exhibit similar decomposition products (Eisentraut et al. 2018). Thus, analysis of NR in environmental samples may be problematic if such extraneous organics cannot be excluded. Styrene-butadiene rubber (SBR), a synthetic petroleum-based product, has also received attention as a chemical marker compound. SBR is abundant in tires, exhibits limited leaching, and has few non-tire sources (Eisentraut et al. 2018).
Sommer et al. (2018) used multiple approaches to identify tire-derived aerosol particulates collected near German roadways. These included transmitted light microscopy and SEM-EDX, as well as diagnostic particle axial ratios and volumes. The authors noted that most tire wear particles exhibited rounded, elongated shapes, with adhering road and brake wear particles. Mengistu et al. (2019) developed a method for detection and quantification of tire wear particles in sediments that entails the use of FTIR, simultaneous thermal analysis (STA), and parallel factor analysis (PARAFAC). STA and FTIR were first used to generate data matrices, which then provided data for the PARAFAC. PARAFAC was used to decompose the overlying components in the spectral data into groups of substances for easier analysis. Mengistu et al. (2019) speculated that with further development and incorporation of PARAFAC with FTIR analysis, the method proposed could be automated for faster analysis of tire wear particles in sediment samples.
2.7 Quality Assurance and Quality Control
The development of appropriate goals and hypotheses is the first and most critical step in plastic debris studies. However, to date many published works have relied on opportunistic field studies or biased sampling designs. This is due in part to the contemporaneous inception of plastics manufacturing (circa 1950) and the recent discovery of the widespread environmental distribution of microplastics. The significance of results must be evaluated in the context of the quality and extent of the sampling, accuracy, and inclusiveness of the analysis methods applied.
de Ruijter et al. (2020) discussed important quality criteria for evaluating microplastic risk assessments. Among those most pertinent to analytical studies were delineating particle size, shape, polymer type, source of microplastic , appropriate reporting units, chemical purity, effectiveness of lab preparation steps, and verification of background contamination and replication. The selection of appropriate study materials to meet study goals is essential. On account of the wide range of plastics made, care must be taken when extrapolating findings from a small subset of plastics to the wide range in commerce. In some lab studies, the basis for polymer types chosen includes concordance with those used in previous studies (a standardization mindset) or their facile acquisition (i.e., commercially available or already in-hand materials). For example, low- and high-density polyethylenes (LDPE and HDPE, respectively) are common in surface waters. However, ultrahigh molecular weight (UHMW) PE has been used as a representative polymer in some influential studies (e.g., Teuten et al. 2007; Bakir et al. 2012) to evaluate microplastic sorption of water-borne organic pollutants. However, UHMWPE is a high crystallinity polymer. It is used in niche applications such as surgical implants in humans and high-precision mechanical gears due to its extreme hardness, inertness, and durability. UHMWPE’s prevalence in the environment is likely very low. Its environmental behavior, versus widely used LDPE and HDPE, merits examination. Additive and filler packages may also differ. Unfortunately, plastic manufacturers often provide an incomplete list of ingredients of plastic products or declare composition “confidential business information.” Accordingly, researchers on occasion have published lab exposure or behavior studies without describing the chemical composition (polymer type or additives present) of the microplastics used (e.g., Ogonowski et al. 2016; Barboza et al. 2018a, b; Pacheco et al. 2018; Martins and Guilhermino 2018). This makes the applicability of results uncertain. Hildebrandt et al. (2019) cautioned that the actual size ranges of commercial microplastic products may differ from supplier specifications and should be verified before use in recovery exercises. They also noted the use of nonspherical microplastics would be more germane in validation exercises, as secondary microplastics are most abundant in the environment.
Contamination of samples during collection and analysis is problematic due to the ubiquitous presence of plastics, especially indoors (e.g., Wesch et al. 2017; Catarino et al. 2018). Where feasible, sampling equipment, materials/reagents used in preparation, and storage containers should be free of plastic components to reduce potential sample contamination. Nets and lines commonly consist of polymeric (e.g., PE, PP, and PA) materials, which may shed fragments (Welden and Cowie 2017). The use of natural fiber sampling nets is an option. Glass containers and metal sieves may be used. However, container closures (e.g., caps) may be a source of microplastic contamination, as shown for bottled water (Winkler et al. 2019). Cotton or non-shedding clothes are recommended for persons collecting and analyzing samples. Nylon is a common component of clothes, protective apparel (e.g., gloves), and water purification apparatuses. Airborne microplastics are very abundant indoors (Catarino et al. 2018) where lab preparation occurs. Hence, samples should be covered where possible. Wesch et al. (2017) noted that the use of a clean bench with particle filtration reduced sample contamination by >96%. Harsh sample treatment (e.g., exposure to abrasion and caustics) may reduce analytical interferences, allowing better spectra to be obtained, but can fragment particles, altering the sample’s original size distribution.
Inclusion of lab and field blanks, as well as positive controls (amending matrices with microplastic standards representing the targeted polymer types, sizes, and shape characteristics) is necessary for quantitative approaches. These should be passed through all procedural steps to evaluate possible contamination and analyte losses (Koelmans et al. 2019). Blank results should be reported. The use of multiple devices or containers during sample processing, exposure to particle-laden ambient air, and addition of preservatives increase the potential for contaminant introduction. Reagents (e.g., oxidizers and preservatives) may be passed through filters to reduce particulates. But the filters themselves must not introduce interferences, and laboratory blanks should be inspected (Koelmans et al. 2019). For example, Fortin et al. (2019) suggested that some sub-10 μm microplastics detected in highly treated wastewater effluent samples evaluated may have arisen from polymeric materials used in the purification equipment itself.
Calls have been made to standardize microplastic sampling and analysis methods to facilitate comparisons between studies. However, sampling and analytical approaches for microplastics remain a “work in progress.” Thus, coalescing on immature methods that fail to adequately identify the range of microplastics is ill-advised. Provencher et al. (2020) discussed the concept of method harmonization versus strict standardization. These authors also point out the responsibility of manuscript reviewers and journal editors in ensuring the appropriateness of the methods, data, and language used by authors.
The question of units for expressing microplastic measurements is critical. Commonly, studies simply report the number of a limited range of particle sizes (e.g., those between 300 and 5000 μm) present. However, this may be misleading (Simon et al. 2018; Rivers et al. 2019). As discussed previously, particle size characteristics are subject to change (i.e., plastic debris fragments over time into smaller particles). Importantly, all enumerations to date are likely underestimates due to a failure to report small micro- and nanoplastics. Studies should strive to determine and report mass-based concentrations of the plastics present. Masses may be estimated by determining particle volumes (e.g., by flow cytometry), although a lack of density measurements will be a source of error in such calculations. However, most polymer densities reside within a range of only about 10%. Alternatively, areas of particles derived from microspectroscopic assessments could be determined and summed by polymer type. Such area totals, as well as estimated volumes and associated masses, could then be calculated (Simon et al. 2018). This would be a significant step forward towards determining mass balances of MPs in the environment.
2.8 Conclusion
Over the last 70 years, plastic debris has entered all global environmental compartments, resulting in the formation of complex mixtures of chemically and physically distinct particles. This reality poses extraordinary sampling and analysis challenges. The researcher’s first and most critical task is to formulate appropriate study goals. This is essential, as no single currently available sampling or analysis protocol is capable of capturing, identifying, and quantitating the full range of plastic debris present (Fig. 2.1; Table 2.1). To date, polymer analysis priorities have largely been guided by plastic production statistics, preexisting data on debris distribution (often skewed towards selected locales, such as beaches and the water’s surface), and ease of sampling and detection. This has begun to change.
Risk at the individual, population, and ecosystem levels should be a driving force for studies and thus for prioritizing analysis method development. But such data remain incomplete. The study of impacts may be well served by controlled exposures. However, even in lab studies, greater diligence regarding the identities and properties of the study materials is warranted (Kögel et al. 2020). The first step to investigate environmental plastic pollution, field sampling, is critical. Careful preparation and highly sophisticated detection instrumentation cannot rescue what inappropriate sampling has missed or compromised. Researchers must recognize that plastic debris in the environment is composed of an immense diversity of polymers of varying properties. Polymers are also commonly augmented with additives or fillers, contributing up to percent by weight of the final plastic product. Their presence may affect plastic debris toxic potential and environmental behavior, as well as their recovery during sampling and subsequent sample preparation (and responses during instrumental detection). Size, shape, and surface texture of debris also affect the behavior in the field and during analysis. Small micro- (<20 μm) and nanoplastics are particularly difficult to characterize and enumerate and accordingly have been rarely reported (Kögel et al. 2020). Ironically, these are likely the most abundant, in part due to the continuous fragmentation of plastic debris in the environment.
The recognition that small microplastics are important is driving sampling towards more inclusive approaches, e.g., the use of pumps and filter arrays to capture and separate them into size classes. However, this may come at the cost of extended analysis time and representative sampling (due to a small sample size). Techniques such as continuous flow centrifugation are promising in this arena. Automation is desirable from the perspective of analysis cost, accuracy, and precision. Complex, labor-intensive treatment schemes also increase the opportunity for analyte loss and sample contamination.
Powerful analytical techniques (e.g., MALDI-MS and AFM) to probe the surface properties of plastics exist. However, these are expensive and inappropriate to quantify large numbers of microplastic targets. μFTIR is becoming more available and is amenable to the automation of particle searching. FPA detectors further reduce analysis time. However, conventional FTIR does not resolve particles below about 10 μm. μRaman has capabilities to detect smaller particles (<1 μm) but is more expensive, time-consuming, and vulnerable to interference from fluorescence. In terms of evaluating total plastic in samples, approaches such as solvent extraction and thermal analysis hold promise. They can encompass submicron plastic debris, but their qualitative power is limited unless enhanced with supplemental techniques. By themselves, they do not provide plastic debris size, shape, or texture data, although sample pre-sieving permits separation into particle size bins. But this, in turn, increases the number of samples to be analyzed. However, these techniques may be readily automated and, compared to more sophisticated approaches, are inexpensive and rugged.
As daunting as the above seems, researchers concerned about plastics in the environment may be able to take advantage of some, to date, largely untapped resources, i.e., expertise and research in other disciplines. These include polymer chemists in the academic and commercial fields, the professionals who actually design and formulate plastics. As such, they possess substantial experience characterizing polymers, as well as knowledge of their behavior. Unfortunately manufacturers often deem compositional details of plastic products confidential. This is a complex issue, involving legal and business concerns (i.e., trade secrets). In some cases these may be fundamentally less important than associated toxicological issues. However, suggestions to remedy this are beyond the scope of this analytical chemistry-tasked chapter. Plastics are engineered to fulfill the performance requirements of specific applications at as low a cost as feasible, maximizing financial returns. Regrettably, postconsumer environmental safety and fate have not always been adequately evaluated and incorporated into the calculation. These considerations must evolve if we are to successfully tackle growing global plastic contamination. Bioplastics are also entering the market in increased volumes, due to concern over finite fossil fuels and the expectation that these materials are more “eco-friendly.” This expands the diversity of analytes and their similarities to naturally occurring polymeric materials present further challenges for analytical methods.
References
Anderson JO, Thundiyil JG, Stolbach A (2012) Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol 8(2):166–175. https://doi.org/10.1007/s13181-011-0203-1
Bakir A, Rowland SJ, Thompson RC (2012) Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar Pollut Bull 64(12):2782–2789. https://doi.org/10.1016/j.marpolbul.2012.09.010
Barboza LGA, Vieira LR, Branco V, Carvalho C, Guilhermino L (2018a) Microplastics increase mercury bioconcentration in gills and bioaccumulation in the liver, and cause oxidative stress and damage in Dicentrarchus labrax juveniles. Sci Rep 8(1):15655. https://doi.org/10.1038/s41598-018-34125-z
Barboza LGA, Vieira LR, Branco V, Figueiredo N, Carvalho F, Carvalho C, Guilhermino L (2018b) Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat Toxicol 195:49–57. https://doi.org/10.1016/j.aquatox.2017.12.008
Bergmann M, Wirzberger V, Krumpen T, Lorenz C, Primpke S, Tekman MB, Gerdts G (2017) High quantities of microplastic in Arctic deep-sea sediments from the HAUSGARTEN observatory. Environ Sci Technol 51(19):11000–11010. https://doi.org/10.1021/acs.est.7b03331
Berto D, Rampazzo F, Gion C, Noventa S, Ronchi F, Traldi U, Giorgi G, Cicero AM, Giovanardi O (2017) Preliminary study to characterize plastic polymers using elemental analyser/isotope ratio mass spectrometry (EA/IRMS). Chemosphere 176:47–56. https://doi.org/10.1016/j.chemosphere.2017.02.090
Biginagwa FJ, Mayoma BS, Shashoua Y, Syberg K, Khan FR (2016) First evidence of microplastics in the African Great Lakes: recovery from Lake Victoria Nile perch and Nile tilapia. J Great Lakes Res 42(1):146–149. https://doi.org/10.1016/j.jglr.2015.10.012
Brahney J, Hallerud M, Heim E, Hahnenberger M, Sukumaran S (2020) Plastic rain in protected areas of the United States. Science 368(6496):1257–1260. https://doi.org/10.1126/science.aaz5819
Brandon JA, Jones W, Ohman MD (2019) Multidecadal increase in plastic particles in coastal ocean sediments. Science Advances, 5(9), eaax0587. https://doi.org/10.1126/sciadv.aax0587
Brenna JT, Creasy WR (1991) High-molecular-weight polymer analysis by laser microprobe Fourier transform ion cyclotron resonance mass spectrometry. Appl Spectrosc 45(1):80–91. https://doi.org/10.1366/0003702914337786
Cabernard L, Roscher L, Lorenz C, Gerdts G, Primpke S (2018) Comparison of Raman and Fourier transform infrared spectroscopy for the quantification of microplastics in the aquatic environment. Environ Sci Technol 52(22):13279–13288. https://doi.org/10.1021/acs.est.8b03438
Cai L, Wang J, Peng J, Wu Z, Tan X (2018) Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci Total Environ 628–629:740–747. https://doi.org/10.1016/j.scitotenv.2018.02.079
Catarino AI, Macchia V, Sanderson WG, Thompson RC, Henry TB (2018) Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ Pollut 237:675–684. https://doi.org/10.1016/j.envpol.2018.02.069
Ceccarini A, Corti A, Erba F, Modugno F, La Nasa J, Bianchi S, Castelvetro V (2018) The hidden microplastics: new insights and figures from the thorough separation and characterization of microplastics and of their degradation byproducts in coastal sediments. Environ Sci Technol 52(10):5634–5643. https://doi.org/10.1021/acs.est.8b01487
Chen H, Hou A, Corilo YE, Lin Q, Lu J, Mendelssohn IA, Zhang R, Rodgers RP, McKenna AM (2016) 4 years after the Deepwater Horizon spill: molecular transformation of Macondo well oil in Louisiana salt marsh sediments revealed by FT-ICR mass spectrometry. Environ Sci Technol 50(17):9061–9069. https://doi.org/10.1021/acs.est.6b01156
Chen Y, Wen D, Pei J, Fei Y, Ouyang D, Zhang H, Luo Y (2020) Identification and quantification of microplastics using Fourier-transform infrared spectroscopy: current status and future prospects. Curr Opin Environ Sci Health 18:14–19. https://doi.org/10.1016/j.coesh.2020.05.004
Cheng H, Hu Y, Reinhard M (2014) Environmental and health impacts of artificial turf: a review. Environ Sci Technol 48:2114–2129. https://doi.org/10.1021/es4044193
Choy CA, Robison BH, Gagne TO, Erwin B, Firl E, Halden RU, Hamilton JA, Katija K, Lisin SE, Rolsky C, Van Houtan KS (2019) The vertical distribution and biological transport of marine microplastics across the epipelagic and mesopelagic water column. Sci Rep 9(1):7843. https://doi.org/10.1038/s41598-019-44117-2
Christie RM (1994) Pigments, dyes and fluorescent brightening agents for plastics: an overview. Polym Int 34(4):351–361. https://doi.org/10.1002/pi.1994.210340401
Courtene-Jones W, Quinn B, Murphy F, Gary SF, Narayanaswamy BE (2017) Optimisation of enzymatic digestion and validation of specimen preservation methods for the analysis of ingested microplastics. Anal Methods 9(9):1437–1445. https://doi.org/10.1039/c6ay02343f
Covernton GA, Pearce CM, Gurney-Smith HJ, Chastain SG, Ross PS, Dower JF, Dudas SE (2019) Size and shape matter: a preliminary analysis of microplastic sampling technique in seawater studies with implications for ecological risk assessment. Sci Total Environ 667:124–132. https://doi.org/10.1016/j.scitotenv.2019.02.346
Dai ZF, Zhang HB, Zhou Q, Tian Y, Chen T, Tu C, Fu CC, Luo YM (2018) Occurrence of microplastics in the water column and sediment in an inland sea affected by intensive anthropogenic activities. Environ Pollut 242:1557–1565. https://doi.org/10.1016/j.envpol.2018.07.131
Dawson AL, Kawaguchi S, King CK, Townsend KA, King R, Huston WM, Bengtson Nash SM (2018) Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat Commun 9(1):1001. https://doi.org/10.1038/s41467-018-03465-9
Dazzi A, Prater C, Hu Q, Chase DB (2012) AFM-IR: combining atomic force microscopy and infrared spectroscopy for nanoscale chemical characterization. Appl Spectrosc 66(12):1365–1384. https://doi.org/10.1366/12-06804
de Ruijter VN, Redondo-Hasselerharm PE, Gouin T, Koelmans AA (2020) Quality criteria for microplastic effect studies in the context of risk assessment: A critical review. Environ Sci Technol 54(19). https://doi.org/10.1021/acs.est.0c03057
Dehghani S, Moore F, Akhbarizadeh R (2017) Microplastic pollution in deposited urban dust, Tehran metropolis, Iran. Environ Sci Pollut Res 24(25):20360–20371. https://doi.org/10.1007/s11356-017-9674-1
Doyen P, Hermabessiere L, Dehaut A, Himber C, Decodts M, Degraeve T, Delord L, Gaboriaud M, Moné P, Sacco J, Tavernier E, Grard T, Duflos G (2019) Occurrence and identification of microplastics in beach sediments from the Hauts-de-France region. Environ Sci Pollut Res 26(27):28010–28021. https://doi.org/10.1007/s11356-019-06027-8
Dris R, Gasperi J, Rocher V, Tassin B (2018) Synthetic and non-synthetic anthropogenic fibers in a river under the impact of Paris megacity: sampling methodological aspects and flux estimations. Sci Total Environ 618:157–164. https://doi.org/10.1016/j.scitotenv.2017.11.009
Duemichen E, Eisentraut P, Celina M, Braun U (2019) Automated thermal extraction-desorption gas chromatography mass spectrometry: A multifunctional tool for comprehensive characterization of polymers and their degradation products. J Chromatogr A 1592:133–142. https://doi.org/10.1016/j.chroma.2019.01.033
Dümichen E, Barthel AK, Braun U, Bannick CG, Brand K, Jekel M, Senz R (2015) Analysis of polyethylene microplastics in environmental samples, using a thermal decomposition method. Water Res 85:451–457. https://doi.org/10.1016/j.watres.2015.09.002
Efimova I, Bagaeva M, Bagaev A, Kileso A, Chubarenko IP (2018) Secondary microplastics generation in the sea swash zone with coarse bottom sediments: laboratory experiments. Front Mar Sci 5. https://doi.org/10.3389/fmars.2018.00313
Eisentraut P, Dümichen E, Ruhl AS, Jekel M, Albrecht M, Gehde M, Braun U (2018) Two birds with one stone – fast and simultaneous analysis of microplastics: microparticles derived from thermoplastics and tire wear. Environ Sci Technol Lett 5(10):608–613. https://doi.org/10.1021/acs.estlett.8b00446
Elert AM, Becker R, Duemichen E, Eisentraut P, Falkenhagen J, Sturm H, Braun U (2017) Comparison of different methods for MP detection: what can we learn from them, and why asking the right question before measurements matters? Environ Pollut 231(Pt 2):1256–1264. https://doi.org/10.1016/j.envpol.2017.08.074
Enders K, Lenz R, Stedmon CA, Nielsen TG (2015) Abundance, size and polymer composition of marine microplastics ≥ 10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar Pollut Bull 100(1):70–81. https://doi.org/10.1016/j.marpolbul.2015.09.027
Erni-Cassola G, Gibson MI, Thompson RC, Christie-Oleza JA (2017) Lost, but found with Nile red: A novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples. Environ Sci Technol 51(23):13641–13648. https://doi.org/10.1021/acs.est.7b04512
Erni-Cassola G, Zadjelovic V, Gibson MI, Christie-Oleza JA (2019) Distribution of plastic polymer types in the marine environment; A meta-analysis. J Hazard Mater 369:691–698. https://doi.org/10.1016/j.jhazmat.2019.02.067
Feng LJ, Wang JJ, Liu SC, Sun XD, Yuan XZ, Wang SG (2018) Role of extracellular polymeric substances in the acute inhibition of activated sludge by polystyrene nanoparticles. Environ Pollut 238:859–865. https://doi.org/10.1016/j.envpol.2018.03.101
Fischer M, Scholz-Böttcher BM (2017) Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography-mass spectrometry. Environ Sci Technol 51(9):5052–5060. https://doi.org/10.1021/acs.est.6b06362
Fortin S, Song B, Burbage C (2019) Quantifying and identifying microplastics in the effluent of advanced wastewater treatment systems using Raman microspectroscopy. Mar Pollut Bull 149:110579. https://doi.org/10.1016/j.marpolbul.2019.110579
Fries E, Dekiff JH, Willmeyer J, Nuelle MT, Ebert M, Remy D (2013) Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ Sci Process Impacts 15(10):1949–1956. https://doi.org/10.1039/c3em00214d
Fu W, Min J, Jiang W, Li Y, Zhang W (2020) Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci Total Environ 721:137561. https://doi.org/10.1016/j.scitotenv.2020.137561
Fuller S, Gautam A (2016) A procedure for measuring microplastics using pressurized fluid extraction. Environ Sci Technol 50(11):5774–5780. https://doi.org/10.1021/acs.est.6b00816
Gasperi J, Wright SL, Dris R, Collard F, Mandin C, Guerrouache M, Langlois V, Kelly FJ, Tassin B (2018) Microplastics in air: are we breathing it in? Curr Opin Environ Sci Health 1:1–5. https://doi.org/10.1016/j.coesh.2017.10.002
GESAMP (2019) Guidelines for the monitoring and assessment of plastic litter and microplastics in the ocean. GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection, London. https://environmentlive.unep.org/media/docs/marine_plastics/une_science_dvision_gesamp_reports.pdf
Ghosal S, Wagner J (2013) Correlated Raman micro-spectroscopy and scanning electron microscopy analyses of flame retardants in environmental samples: A micro-analytical tool for probing chemical composition, origin and spatial distribution. Analyst 138(13):3836–3844. https://doi.org/10.1039/c3an00501a
Gillibert R, Balakrishnan G, Deshoules Q, Tardivel M, Magazzù A, Donato MG, Maragò OM, Lamy de La Chapelle M, Colas F, Lagarde F, Gucciardi PG (2019) Raman tweezers for small microplastics and nanoplastics identification in seawater. Environ Sci Technol 53(15):9003–9013. https://doi.org/10.1021/acs.est.9b03105
Girão AV, Caputo G, Ferro MC (2017) Application of scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS). Compr Anal Chem 75:153–168. https://doi.org/10.1016/bs.coac.2016.10.002
Gniadek M, Dąbrowska A (2019) The marine nano- and microplastics characterisation by SEM-EDX: the potential of the method in comparison with various physical and chemical approaches. Mar Pollut Bull 148:210–216. https://doi.org/10.1016/j.marpolbul.2019.07.067
Gomiero A, Øysæd KB, Agustsson T, van Hoytema N, van Thiel T, Grati F (2019) First record of characterization, concentration and distribution of microplastics in coastal sediments of an urban fjord in Southwest Norway using a thermal degradation method. Chemosphere 227:705–714. https://doi.org/10.1016/j.chemosphere.2019.04.096
Haave M, Lorenz C, Primpke S, Gerdts G (2019) Different stories told by small and large microplastics in sediment – first report of microplastic concentrations in an urban recipient in Norway. Mar Pollut Bull 14:501–513. https://doi.org/10.1016/j.marpolbul.2019.02.015
Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P (2018) An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater 344:179–199. https://doi.org/10.1016/j.jhazmat.2017.10.014
Hakkarainen M (2012) Mass spectrometry of polymers – new techniques. Springer, Berlin/Heidelberg, 212p. https://doi.org/10.1007/978-3-642-28041-2
Hale RC (2017) Analytical challenges associated with the determination of microplastics in the environment. Anal Methods 9:1326–1327. https://doi.org/10.1039/c7ay90015e
Hale RC (2018) Are the risks from microplastics truly trivial? Environ Sci Technol 52(3):931. https://doi.org/10.1021/acs.est.7b06615
Hale RC, La Guardia MJ, Harvey E, Matt Mainor T (2002) Potential role of fire retardant-treated polyurethane foam as a source of brominated diphenyl ethers to the US environment. Chemosphere 46(5):729–735. https://doi.org/10.1016/S0045-6535(01)00237-5
Hale RC, Seeley ME, La Guardia MJ, Mai L, Zeng EY (2020) A global perspective on microplastics. J Geophys Res Oceans 125(1):1–40. https://doi.org/10.1029/2018jc014719
Harrison JP, Ojeda JJ, Romero-González ME (2012) The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Sci Total Environ 416:455–463. https://doi.org/10.1016/j.scitotenv.2011.11.078
Hendrickson E, Minor EC, Schreiner K (2018) Microplastic abundance and composition in western Lake Superior as determined via microscopy, Pyr-GC/MS, and FTIR. Environ Sci Technol 52(4):1787–1796. https://doi.org/10.1021/acs.est.7b05829
Hermabessiere L, Dehaut A, Paul-Pont I, Lacroix C, Jezequel R, Soudant P, Duflos G (2017) Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 182:781–793. https://doi.org/10.1016/j.chemosphere.2017.05.096
Hernandez LM, Yousefi N, Tufenkji N (2017) Are there nanoplastics in your personal care products? Environ Sci Technol Lett 4(7):280–285. https://doi.org/10.1021/acs.estlett.7b00187
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ Sci Technol 46(6):3060–3075. https://doi.org/10.1021/es2031505
Hildebrandt NL, Voigta N, Zimmermann T, Reese A, Proefrock D (2019) Evaluation of continuous flow centrifugation as an alternative technique to sample microplastic from water bodies. Mar Environ Res 151:104768. https://doi.org/10.1016/j.marenvres.2019.104768
Hildebrandt L, Mitrano DM, Zimmermann T, Pröfrock D (2020) A nanoplastic sampling and enrichment approach by flow centrifugation. Front Environ Sci 8:89. https://doi.org/10.3389/fenvs.2020.00089
Hintersteiner I, Himmelsbach M, Buchberger WW (2015) Characterization and quantitation of polyolefin microplastics in personal-care products using high-temperature gel-permeation chromatography. Anal Bioanal Chem 407(4):1253–1259. https://doi.org/10.1007/s00216-014-8318-2
Huppertsberg S, Knepper TP (2018) Instrumental analysis of microplastics—benefits and challenges. Anal Bioanal Chem 410(25):6343–6352. https://doi.org/10.1007/s00216-018-1210-8
Hurley RR, Lusher AL, Olsen M, Nizzetto L (2018) Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ Sci Technol 52:7409–7417. https://doi.org/10.1021/acs.est.8b01517
Imhof HK, Schmid J, Niessner R, Ivleva NP, Laforsch C (2012) A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol Oceanogr Methods 10(7):524–537. https://doi.org/10.4319/lom.2012.10.524
Imhof HK, Laforsch C, Wiesheu AC, Schmid J, Anger PM, Niessner R, Ivleva NP (2016) Pigments and plastic in limnetic ecosystems: A qualitative and quantitative study on microparticles of different size classes. Water Res 98:64–74. https://doi.org/10.1016/j.watres.2016.03.015
Jungnickel H, Pund R, Tentschert J, Reichardt P, Laux P, Harbach H, Luch A (2016) Time-of-flight secondary ion mass spectrometry (ToF-SIMS)-based analysis and imaging of polyethylene microplastics formation during sea surf simulation. Sci Total Environ 563–564:261–266. https://doi.org/10.1016/j.scitotenv.2016.04.025
Kane IA, Clare MA, Miramontes E, Wogelius R, Rothwell JJ, Garreau P, Pohl F (2020) Seafloor microplastic hotspots controlled by deep-sea circulation. Science 368(6495):1140–1145. https://doi.org/10.1126/science.aba5899
Käppler A, Windrich F, Löder MGJ, Malanin M, Fischer D, Labrenz M, Eichhorn KJ, Voit B (2015) Identification of microplastics by FTIR and Raman microscopy: a novel silicon filter substrate opens the important spectral range below 1300 cm−1 for FTIR transmission measurements. Anal Bioanal Chem 407(22):6791–6801
Käppler A, Fischer D, Oberbeckmann S, Schernewski G, Labrenz M, Eichhorn K-J, Voit B (2016) Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal Bioanal Chem 408(29):8377–8391. https://doi.org/10.1007/s00216-016-9956-3
Karami A, Golieskardi A, Choo CK, Romano N, Ho YB, Salamatinia B (2017) A high-performance protocol for extraction of microplastics in fish. Sci Total Environ 578:485–494. https://doi.org/10.1016/j.scitotenv.2016.10.213
Khaled A, Rivaton A, Richard C, Jaber F, Sleiman M (2018) Phototransformation of plastic containing brominated flame retardants: enhanced fragmentation and release of photoproducts to water and air. Environ Sci Technol 52(19):11123–11131. https://doi.org/10.1021/acs.est.8b03172
Klein S, Dimzon IK, Eubeler J, Knepper TP (2018) Analysis, occurrence, and degradation of microplastics in the aqueous environment. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Cham, pp 51–67
Klöckner P, Reemtsma T, Eisentraut P, Braun U, Ruhl AS, Wagner S (2019) Tire and road wear particles in road environment – quantification and assessment of particle dynamics by Zn determination after density separation. Chemosphere 222:714–721. https://doi.org/10.1016/j.chemosphere.2019.01.176
Koelmans AA, Mohamed Nor NH, Hermsen E, Kooi M, Mintenig SM, De France J (2019) Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res 155:410–422. https://doi.org/10.1016/j.watres.2019.02.054
Kögel T, Bjorøy Ø, Toto B, Bienfait AM, Sanden M (2020) Micro- and nanoplastic toxicity on aquatic life: determining factors. Sci Total Environ 709(5817):136050. https://doi.org/10.1016/j.scitotenv.2019.136050
Kole PJ, Löhr AJ, Van Belleghem FGAJ, Raga AMJ (2017) Wear and tear of tyres: A stealthy source of microplastics in the environment. Int J Environ Res Public Health 14(10):1265. https://doi.org/10.3390/ijerph14101265
Kooi M, Nes E Hv, Scheffer M, Koelmans AA (2017) Ups and downs in the ocean: effects of biofouling on vertical transport of microplastics. Environ Sci Technol 51(14):7963–7971. https://doi.org/10.1021/acs.est.6b04702
Lapointe M, Farner JM, Hernandez LM, Tufenkji N (2020) Understanding and improving microplastic removal during water treatment: impact of coagulation and flocculation. Environ Sci Technol 54(14):8719–8727. https://doi.org/10.1021/acs.est.0c00712
Leads RR, Weinstein JE (2019) Occurrence of tire wear particles and other microplastics within the tributaries of the Charleston Harbor estuary, South Carolina, USA. Mar Pollut Bull 145:569–582. https://doi.org/10.1016/j.marpolbul.2019.06.061
Lenz R, Enders K, Stedmon CA, MacKenzie DMA, Nielsen TG (2015) A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar Pollut Bull 100(1):82–91. https://doi.org/10.1016/j.marpolbul.2015.09.026
Leslie HA, Brandsma SH, van Velzen MJM, Vethaak AD (2017) Microplastics en route: field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ Int 101:133–142. https://doi.org/10.1016/j.envint.2017.01.018
Li JN, Green C, Reynolds A, Shi HH, Rotchell JM (2018a) Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ Pollut 241:35–44. https://doi.org/10.1016/j.envpol.2018.05.038
Li JY, Liu HH, Chen JP (2018b) Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res 137:362–374. https://doi.org/10.1016/j.watres.2017.12.056
Li HR, La Guardia MJ, Liu HH, Hale RC, Mainor TM, Harvey E, Sheng GY, Fu JM, Peng PA (2019) Brominated and organophosphate flame retardants along a sediment transect encompassing the Guiyu, China e-waste recycling zone. Sci Total Environ 646:58–67. https://doi.org/10.1016/j.scitotenv.2018.07.276
Lin Y, Huang X, Liu Q, Lin ZY, Jiang GB (2020) Thermal fragmentation enhanced identification and quantification of polystyrene micro/nanoplastics in complex media. Talanta 208:120478. https://doi.org/10.1016/j.talanta.2019.120478
Lithner D, Larsson A, Dave G (2011) Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci Total Environ 409(18):3309–3324. https://doi.org/10.1016/j.scitotenv.2011.04.038
Liu F, Olesen KB, Borregaard AR, Vollertsen J (2019) Microplastics in urban and highway stormwater retention ponds. Sci Total Environ 671. https://doi.org/10.1016/j.scitotenv.2019.03.416
Löder MGJ, Gerdts G (2015) Methodology used for the detection and identification of microplastics—a critical appraisal. In: Bergmann M, Gutow L, Klages M (eds) Marine anthropogenic litter. Springer, Cham, pp 201–227
Löder MGJ, Kuczera M, Mintenig S, Lorenz C, Gerdts G (2015) Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ Chem 12(5):563–581. https://doi.org/10.1071/EN14205
Löder MGJ, Imhof HK, Ladehoff M, Löschel LA, Lorenz C, Mintenig S, Piehl S, Primpke S, Schrank I, Laforsch C, Gerdts G (2017) Enzymatic purification of microplastics in environmental samples. Environ Sci Technol 51(24):14283–14292. https://doi.org/10.1021/acs.est.7b03055
Lu SH, Zhu KR, Song WC, Song G, Chen DY, Hayat T, Alharbi NS, Chen CL, Sun Y (2018) Impact of water chemistry on surface charge and aggregation of polystyrene microspheres suspensions. Sci Total Environ 630:951–959. https://doi.org/10.1016/j.scitotenv.2018.02.296
Luan L, Wang X, Zheng H, Liu L, Luo X, Li F (2019) Differential toxicity of functionalized polystyrene microplastics to clams (Meretrix meretrix) at three key development stages of life history. Mar Pollut Bull 139:346–354. https://doi.org/10.1016/j.marpolbul.2019.01.003
Luo HW, Xiang YH, He DQ, Li Y, Zhao YY, Wang S, Pan XL (2019) Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris. Sci Total Environ 678:1–9. https://doi.org/10.1016/j.scitotenv.2019.04.401
Luo H, Zhao Y, Li Y, Xiang Y, He D, Pan X (2020) Aging of microplastics affects their surface properties, thermal decomposition, additives leaching and interactions in simulated fluid. Sci Total Environ 714:136862. https://doi.org/10.1016/j.scitotenv.2020.136862
Lusher AL, McHugh M, Thompson RC (2013) Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar Pollut Bull 67(1):94–99. https://doi.org/10.1016/j.marpolbul.2012.11.028
Lusher AL, Welden NA, Sobral P, Cole M (2017) Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal Methods 9(9):1346–1360
Maes T, Jessop R, Wellner N, Haupt K, Mayes AG (2017) A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile red. Sci Rep 7:44501. https://doi.org/10.1038/srep44501
Mahon AM, O'Connell B, Healy MG, O'Connor I, Officer R, Nash R, Morrison L (2017) Microplastics in sewage sludge: effects of treatment. Environ Sci Technol 51(2):810–818. https://doi.org/10.1021/acs.est.6b04048
Martins A, Guilhermino L (2018) Transgenerational effects and recovery of microplastics exposure in model populations of the freshwater cladoceran Daphnia magna Straus. Sci Total Environ 631–632:421–428. https://doi.org/10.1016/j.scitotenv.2018.03.054
Materić D, Kasper-Giebl A, Kau D, Anten M, Greilinger M, Ludewig E, van Sebille E, Röckmann T, Holzinger R (2020) Micro- and nanoplastics in alpine snow: A new method for chemical identification and (semi)quantification in the nanogram range. Environ Sci Technol 54(4):2353–2359. https://doi.org/10.1021/acs.est.9b07540
McGivney E, Cederholm L, Barth A, Hakkarainen M, Hamacher-Barth E, Ogonowski M, Gorokhova E (2020) Rapid physicochemical changes in microplastic induced by biofilm formation. Front Bioeng Biotechnol 8:205. https://doi.org/10.3389/fbioe.2020.00205
Mengistu D, Nilsen V, Heistad A, Kvaal K (2019) Detection and quantification of tire particles in sediments using a combination of simultaneous thermal analysis, Fourier transform infra-red, and parallel factor analysis. Int J Environ Res Public Health 16(18):3444. https://doi.org/10.3390/ijerph16183444
Merzel RL, Purser L, Soucy TL, Olszewski M, Colón-Bernal I, Duhaime M, Elgin AK, Banaszak Holl MM (2019) Uptake and retention of nanoplastics in quagga mussels. Global Chall 1800104. https://doi.org/10.1002/gch2.201800104
Michels J, Stippkugel A, Lenz M, Wirtz K, Engel A (2018) Rapid aggregation of biofilm-covered microplastics with marine biogenic particles. Proc R Soc B 285(1885):20181203. https://doi.org/10.1098/rspb.2018.1203
Miller-Chou BA, Koenig JL (2003) A review of polymer dissolution. Prog Polym Sci 28:1223–1270. https://doi.org/10.1016/S0079-6700(03)00045-5
Mintenig SM, Bäuerlein PS, Koelmans AA, Dekker SC, Wezel, A. P. v. (2018) Closing the gap between small and smaller: towards a framework to analyse nano- and microplastics in aqueous environmental samples. Environ Sci Nano 5(7):1640–1649. https://doi.org/10.1039/c8en00186c
Möller JN, Löder MGJ, Laforsch C (2020) Finding microplastics in soils: A review of analytical methods. Environ Sci Technol 54(4):2078–2090. https://doi.org/10.1021/acs.est.9b04618
Munno K, De Frond H, O’Donnell B, Rochman CM (2020) Increasing the accessibility for characterizing microplastics: introducing new application-based and spectral libraries of plastic particles (SLoPP and SLoPP-E). Anal Chem 92(3):2443–2451. https://doi.org/10.1021/acs.analchem.9b03626
Nakajima R, Lindsay DJ, Tsuchiya M, Matsui R, Kitahashi T, Fujikura K, Fukushima T (2019a) A small, stainless-steel sieve optimized for laboratory beaker-based extraction of microplastics from environmental samples. MethodsX 6:1677–1682. https://doi.org/10.1016/j.mex.2019.07.012
Nakajima R, Tsuchiya M, Lindsay DJ, Kitahashi T, Fujikura K, Fukushima T (2019b) A new small device made of glass for separating microplastics from marine and freshwater sediments. PeerJ 7:e7915. https://doi.org/10.7717/peerj.7915
Ng KL, Obbard JP (2006) Prevalence of microplastics in Singapore's coastal marine environment. Marine Pollut Bull 52(7):761–767. https://doi.org/10.1016/j.marpolbul.2005.11.017
Ng EL, Huerta Lwanga E, Eldridge SM, Johnston P, Hu HW, Geissen V, Chen D (2018) An overview of microplastic and nanoplastic pollution in agroecosystems. Sci Total Environ 627:1377–1388. https://doi.org/10.1016/j.scitotenv.2018.01.341
Obbard RW, Sadri S, Wong YQ, Khitun AA, Baker I, Thompson RC (2014) Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth’s Future 2(6):315–320. https://doi.org/10.1002/2014ef000240
Ogonowski M, Schür C, Jarsén Å, Gorokhova E (2016) The effects of natural and anthropogenic microparticles on individual fitness in Daphnia magna. PLoS One 11(5):e0155063. https://doi.org/10.1371/journal.pone.0155063
Oßmann BE, Sarau G, Schmitt SW, Holtmannspötter H, Christiansen SH, Dicke W (2017) Development of an optimal filter substrate for the identification of small microplastic particles in food by micro-Raman spectroscopy. Anal Bioanal Chem 409(16):4099–4109. https://doi.org/10.1007/s00216-017-0358-y
Pabortsava K, Lampitt RS (2020) High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat Commun 11:4073. https://doi.org/10.1038/s41467-020-17932-9
Pacheco A, Martins A, Guilhermino L (2018) Toxicological interactions induced by chronic exposure to gold nanoparticles and microplastics mixtures in Daphnia magna. Sci Total Environ 628–629:474–483. https://doi.org/10.1016/j.scitotenv.2018.02.081
Peñalver R, Arroyo-Manzanares N, López-García I, Hernández-Córdoba M (2020) An overview of microplastics characterization by thermal analysis. Chemosphere 242:125170. https://doi.org/10.1016/j.chemosphere.2019.125170
Peters CA, Hendrickson E, Minor EC, Schreiner K, Halbur J, Bratton SP (2018) Pyr-GC/MS analysis of microplastics extracted from the stomach content of benthivore fish from the Texas Gulf Coast. Mar Pollut Bull 137:91–95. https://doi.org/10.1016/j.marpolbul.2018.09.049
Prata JC, da Costa JP, Duarte AC, Rocha-Santos T (2019) Methods for sampling and detection of microplastics in water and sediment: A critical review. Trends Anal Chem 110:150–159. https://doi.org/10.1016/j.trac.2018.10.029
Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T (2020) Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ 702:134455. https://doi.org/10.1016/j.scitotenv.2019.134455
Primpke S, Lorenz C, Rascher-Friesenhausen R, Gerdts G (2017) An automated approach for microplastics analysis using focal plane array (FPA) FTIR microscopy and image analysis. Anal Methods 9(9):1499–1511. https://doi.org/10.1039/c6ay02476a
Primpke S, Wirth M, Lorenz C, Gerdts G (2018) Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Anal Bioanal Chem 410(21):5131–5141. https://doi.org/10.1007/s00216-018-1156-x
Primpke S, Dias PA, Gerdts G (2019) Automated identification and quantification of microfibres and microplastics. Anal Methods 16:2138–2147. https://doi.org/10.1039/C9AY00126C
Provencher JF, Covernton GA, Moore RC, Horn DA, Conkle JA, Lusher AL (2020) Proceed with caution: the need to raise the publication bar for microplastics research. Sci Total Environ 748:141426. https://doi.org/10.1016/j.scitotenv.2020.141426
Quang TN, He C, Morawska L, Knibbs LD, Falk M (2012) Vertical particle concentration profiles around urban office buildings. Atmos Chem Phys 12(11):5017–5030. https://doi.org/10.5194/acp-12-5017-2012
Reed S, Clark M, Thompson R, Hughes KA (2018) Microplastics in marine sediments near Rothera Research Station, Antarctica. Mar Pollut Bull 133:460–463. https://doi.org/10.1016/j.marpolbul.2018.05.068
Riedel T, Dittmar T (2014) A method detection limit for the analysis of natural organic matter via Fourier transform ion cyclotron resonance mass spectrometry. Anal Chem 86(16):8376–8382. https://doi.org/10.1021/ac501946m
Rivers ML, Gwinnett C, Woodall LC (2019) Quantification is more than counting: actions required to accurately quantify and report isolated marine microplastics. Mar Pollut Bull 139:100–104. https://doi.org/10.1016/j.marpolbul.2018.12.024
Rochman CM et al (2019) Rethinking microplastics as a diverse contaminant suite. Environ Toxicol Chem 38:703–711. https://doi.org/10.1002/etc.4371
Rodrigues SM, Almeida R, C. M., & Ramos, S. (2019) Adaptation of a laboratory protocol to quantity microplastics contamination in estuarine waters. MethodsX 6:740–749. https://doi.org/10.1016/j.mex.2019.03.027
Rummel CD, Jahnke A, Gorokhova E, Kühnel D, Schmitt-Jansen M (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett 4(7):258–267. https://doi.org/10.1021/acs.estlett.7b00164
Schirinzi GF, Llorca M, Seró R, Moyano E, Barceló D, Abad E, Farré M (2019) Trace analysis of polystyrene microplastics in natural waters. Chemosphere 236:124321. https://doi.org/10.1016/j.chemosphere.2019.07.052
Schür C, Rist S, Baun A, Mayer P, Hartmann NB, Wagner M (2019) When fluorescence is not a particle: the tissue translocation of microplastics in Daphnia magna seems an artifact. Environ Toxicol Chem 38(7):1495–1503. https://doi.org/10.1002/etc.4436
Silva AB, Bastos AS, Justino CIL, da Costa JP, Duarte AC, Rocha-Santos TAP (2018) Microplastics in the environment: challenges in analytical chemistry – A review. Anal Chim Acta 1017:1–19. https://doi.org/10.1016/j.aca.2018.02.043
Simon M, van Alst N, Vollertsen J (2018) Quantification of microplastic mass and removal rates at wastewater treatment plants applying focal plane Array (FPA)- based Fourier transform infrared (FT-IR) imaging. Water Res 142:1–9. https://doi.org/10.1016/j.watres.2018.05.019
Singh N, Duanb H, Tang Y (2020) Toxicity evaluation of E-waste plastics and potential repercussions for human health. Environ Int 137:105559. https://doi.org/10.1016/j.envint.2020.105559
Sommer F, Dietze V, Baum A, Sauer J, Gilge S, Maschowski C, Gieré R (2018) Tire abrasion as a major source of microplastics in the environment. Aerosol Air Qual Res 18:2014–2028. https://doi.org/10.4209/aaqr.2018.03.0099
Song YK, Hong SH, Jang M, Kang J-H, Kwon OY, Han GM, Shim WJ (2014) Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environ Sci Technol 48(16):9014–9021. https://doi.org/10.1021/es501757s
Song YK, Hong SH, Jang M, Han GM, Jung SW, Shim WJ (2017) Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ Sci Technol 51(8):4368–4376. https://doi.org/10.1021/acs.est.6b06155
Spies RB, Andresen BD, Rice DW Jr (1987) Benzthiazoles in estuarine sediments as indicators of street runoff. Nature 327(6124):697–699. https://doi.org/10.1038/327697a0
Su YL, Zhang ZJ, Wu D, Zhan L, Shi HH, Xie B (2019) Occurrence of microplastics in landfill systems and their fate with landfill age. Water Res 164:114968. https://doi.org/10.1016/j.watres.2019.114968
Suaria G, Achtypi A, Perold V, Lee J, Pierucci A, Bornman T, Aliani S, Ryan P (2020) Microfibers in oceanic surface waters: a global characterization. Sci Adv 6(23):8493. https://doi.org/10.1126/sciadv.aay8493
Summers S, Henry T, Gutierrez T (2018) Agglomeration of nano- and microplastic particles in seawater by autochthonous and de novo-produced sources of exopolymeric substances. Mar Pollut Bull 130:258–267. https://doi.org/10.1016/j.marpolbul.2018.03.039
Tagg AS, Sapp M, Harrison JP, Ojeda JJ (2015) Identification and quantification of microplastics in wastewater using focal plane array-based reflectance micro-FT-IR imaging. Anal Chem 87(12):6032–6040. https://doi.org/10.1021/acs.analchem.5b00495
Tagg AS, Harrison JP, Ju-Nam Y, Sapp M, Bradley EL, Sinclair CJ, Ojeda JJ (2017) Fenton's reagent for the rapid and efficient isolation of microplastics from wastewater. Chem Commun 53:372–375. https://doi.org/10.1039/c6cc08798a
Takahashi K (2013) Polymer analysis by supercritical fluid chromatography. J Biosci Bioeng 116(2):133–140. https://doi.org/10.1016/j.jbiosc.2013.02.001
Takahashi CK, Turner A, Millward GE, Glegg GA (2012) Persistence and metallic composition of paint particles in sediments from a tidal inlet. Mar Pollut Bull 64(1):133–137. https://doi.org/10.1016/j.marpolbul.2011.10.010
Tamminga M, Stoewer S-C, Fischer EK (2019) On the representativeness of pump water samples versus manta sampling in microplastic analysis. Environ Pollut 254:112970. https://doi.org/10.1016/j.envpol.2019.112970
Ter Halle A, Jeanneau L, Martignac M, Jardé E, Pedrono B, Brach L, Gigault J (2017) Nanoplastic in the North Atlantic subtropical gyre. Environ Sci Technol 51(23):13689–13697. https://doi.org/10.1021/acs.est.7b03667
Teuten EL, Rowland SJ, Galloway TS, Thompson RC (2007) Potential for plastics to transport hydrophobic contaminants. Environ Sci Technol 41(22):7759–7764. https://doi.org/10.1021/es071737s
Tian LL, Chen QQ, Jiang W, Wang LH, Xie HX, Kalogerakis N, Ma YY, Ji R (2019) A carbon-14 radiotracer-based study on the phototransformation of polystyrene nanoplastics in water versus in air. Environ Sci Nano 6(9):2907–2917. https://doi.org/10.1039/c9en00662a
Turner A (2010) Marine pollution from antifouling paint particles. Mar Pollut Bull 60(2):159–171. https://doi.org/10.1016/j.marpolbul.2009.12.004
Turner A, Wallerstein C, Arnold R (2019) Identification, origin and characteristics of bio-bead microplastics from beaches in western Europe. Sci Total Environ 664:938–947. https://doi.org/10.1016/j.scitotenv.2019.01.281
Unice KM, Kreider ML, Panko JM (2012) Use of a deuterated internal standard with pyrolysis-GC/MS dimeric marker analysis to quantify tire tread particles in the environment. [research support, non-U.S. Gov't]. Int J Environ Res Public Health 9(11):4033–4055. https://doi.org/10.3390/ijerph9114033
Van Cauwenberghe L, Devriese L, Galgani F, Robbens J, Janssen CR (2015) Microplastics in sediments: A review of techniques, occurrence and effects. Marine Environ Res 111:5–17. https://doi.org/10.1016/j.marenvres.2015.06.007
van Sebille E, Wilcox C, Lebreton L, Maximenko N, Hardesty BD, van Franeker JA, Eriksen M, Siegel D, Galgani F, Law KL (2015) A global inventory of small floating plastic debris. Environ Res Lett 10(12):124006. https://doi.org/10.1088/1748-9326/10/12/124006
Vianello A, Jensen RL, Liu L, Vollertsen J (2019) Simulating human exposure to indoor airborne microplastics using a breathing thermal manikin. Sci Rep 9(8670). https://doi.org/10.1038/s41598-019-45054-w
von Moos N, Burkhardt-Holm P, Köhler A (2012) Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol 46(20):11327–11335. https://doi.org/10.1021/es302332w
Wagner S, Hüffer T, Klöckner P, Wehrhahn M, Hofmann T, Reemtsma T (2018) Tire wear particles in the aquatic environment – A review on generation, analysis, occurrence, fate and effects. Water Res 139:83–100. https://doi.org/10.1016/j.watres.2018.03.051
Wang ZM, Wagner J, Ghosal S, Bedi G, Wall S (2017) SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci Total Environ 603–604:616–626. https://doi.org/10.1016/j.scitotenv.2017.06.047
Wang Z, Taylor SE, Sharma P, Flury M (2018) Poor extraction efficiencies of polystyrene nano- and microplastics from biosolids and soil. PLoS One 13(11):e0208009. https://doi.org/10.1371/journal.pone.0208009
Wang J, Liu XH, Liu GN, Zhang ZX, Wu H, Cui BS, Bai JH, Zhang W (2019) Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene. Ecotoxicol Environ Saf 173:331–338. https://doi.org/10.1016/j.ecoenv.2019.02.037
Ward JE, Zhao S, Holohan BA, Mladinich KM, Griffin TW, Wozniak J, Shumway SE (2019) Selective ingestion and egestion of plastic particles by the blue mussel (Mytilus edulis) and eastern oyster (Crassostrea virginica): implications for using bivalves as bioindicators of microplastic pollution. Environ Sci Technol 53:8776–8784. https://doi.org/10.1021/acs.est.9b02073
Weidner SM, Trimpin S (2011) Mass spectrometry of synthetic polymers. Anal Chem 82:4811–4829. https://doi.org/10.1002/9783527641826
Welden NA, Cowie PR (2017) Degradation of common polymer ropes in a sublittoral marine environment. Mar Pollut Bull 118(1):248–253. https://doi.org/10.1016/j.marpolbul.2017.02.072
Wesch C, Elert AM, Wörner M, Braun U, Klein R, Paulus M (2017) Assuring quality in microplastic monitoring: about the value of clean-air devices as essentials for verified data. Sci Rep 7(1):5424. https://doi.org/10.1038/s41598-017-05838-4
Whalley J, Zandi S (2016) Particulate matter sampling techniques and data modelling methods. In: Sallis PJ (ed) Air quality – measurement and modeling. https://doi.org/10.5772/62563
Winkler A, Santo N, Ortenzi MA, Bolzoni E, Bacchetta R, Tremolada P (2019) Does mechanical stress cause microplastic release from plastic water bottles? Water Res 166:115082. https://doi.org/10.1016/j.watres.2019.115082
Wolff S, Kerpen J, Prediger J, Barkmann L, Müller L (2019) Determination of the microplastics emission in the effluent of a municipal waste water treatment plant using Raman microspectroscopy. Water Research X 2:100014. https://doi.org/10.1016/j.wroa.2018.100014
Woods MN, Stack ME, Fields DM, Shaw SD, Matrai PA (2018) Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis). Mar Pollut Bull 137:638–645. https://doi.org/10.1016/j.marpolbul.2018.10.061
Wright SL, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms: A review. Environ Pollut 178:483–492. https://doi.org/10.1016/j.envpol.2013.02.031
Wright SL, Levermore JM, Kelly FJ (2019) Raman spectral imaging for the detection of inhalable microplastics in ambient particulate matter samples. Environ Sci Technol 53(15):8947–8956. https://doi.org/10.1021/acs.est.8b06663
Wu N, Herrmann T, Paepke O, Tickner J, Hale R, Harvey E, La Guardia M, McClean MD, Webster TF (2007) Human exposure to PBDEs: associations of PBDE body burdens with food consumption and house dust concentrations. Environ Sci Technol 41(5):1584–1589. https://doi.org/10.1021/es0620282
Yu XB, Ladewig S, Bao S, Toline CA, Whitmire S, Chow AT (2018) Occurrence and distribution of microplastics at selected coastal sites along the southeastern United States. Sci Total Environ 613–614:298–305. https://doi.org/10.1016/j.scitotenv.2017.09.100
Zettler ER, Mincer TJ, Amaral-Zettler LA (2013) Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol 47(13):7137–7146. https://doi.org/10.1021/es401288x
Zhang Y, Kang S, Allen S, Allen D, Gao T, Sillanpää M (2020a) Atmospheric microplastics: A review on current status and perspectives. Earth Sci Rev 203. https://doi.org/10.1016/j.earscirev.2020.103118
Zhang XM, Mell A, Li F, Thaysen C, Musselman B, Tice J, Vukovic D, Rochman C, Helm PA, Jobst KJ (2020b) Rapid fingerprinting of source and environmental microplastics using direct analysis in real time-high resolution mass spectrometry. Anal Chim Acta 1100:107–117. https://doi.org/10.1016/j.aca.2019.12.005
Zhang K, Hamidian AH, Tubić A, Zhang Y, Fang JKH, Wu C, Lam PKS (2021) Understanding plastic degradation and microplastic formation in the environment: a review. Environ Pollut 274:116554. https://doi.org/10.1016/j.envpol.2021.116554
Acknowledgement
This is Contribution 3906 of the Virginia Institute of Marine Science, William & Mary.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Hale, R.C., Seeley, M.E., King, A.E., Yu, L.H. (2022). Analytical Chemistry of Plastic Debris: Sampling, Methods, and Instrumentation. In: Bank, M.S. (eds) Microplastic in the Environment: Pattern and Process. Environmental Contamination Remediation and Management. Springer, Cham. https://doi.org/10.1007/978-3-030-78627-4_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-78627-4_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-78626-7
Online ISBN: 978-3-030-78627-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)