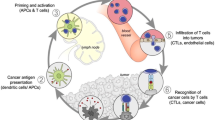
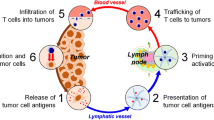
Abstract
In the fight against cancer, studies have shown that administering combination treatments such as chemotherapy and radiotherapy improve patient outcomes. Ultimately to overcome the limitations posed by current treatments, such as deleterious side effects and high reoccurrence rates, new therapeutic strategies need to be explored and developed. Recently, methods designed to modulate the immune system using antigens, adjuvants, and immunotherapy drugs have been approved for use in patients and have shown to increase patients’ survival. For effective delivery of immunoagents, nanoparticles have been explored for their ability to deliver cancer therapeutics. Moreover, plasmonic metal and organic nanoparticles can be multifunctional in their mode of action not only acting as delivery agents of drugs, but also as additional therapeutic vessels since their unique properties enable photothermal and photodynamic therapy. A plethora of studies have demonstrated that chemotherapy, photothermal, and photodynamic therapy can prime the immune system for effective cancer killing and cancer vaccine effects, as immunogenic cell death occurs after administration of these treatments. This chapter highlights various nanoparticle platforms used in the cancer therapeutic arsenal for immunotherapy. Moreover, combination of nanomaterials with other treatments, including photothermal therapy, photodynamic therapy, and chemotherapy will be discussed towards the understanding of defeating cancer and preventing tumor recurrence.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Yarchoan, M., Hopkins, A., Jaffee, E.M.: Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 377(25), 2500 (2017)
Sharma, P., et al.: Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 168(4), 707–723 (2017)
Riley, R.S., et al.: Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov., 1 (2019)
Jeanbart, L., Swartz, M.A.: Engineering opportunities in cancer immunotherapy. Proc. Natl. Acad. Sci. 112(47), 14467–14472 (2015)
Manolova, V., et al.: Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 38(5), 1404–1413 (2008)
Guo, L.R., et al.: Combinatorial photothermal and Immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano. 8(6), 5670–5681 (2014)
Milling, L., Zhang, Y., Irvine, D.J.: Delivering safer immunotherapies for cancer. Adv. Drug Deliv. Rev. 114, 79–101 (2017)
Pfreundschuh, M.G., et al.: Phase I study of intratumoral application of recombinant human tumor necrosis factor. Eur. J. Cancer Clin. Oncol. 25(2), 379–388 (1989)
van Herpen, C.M., et al.: Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int. J. Cancer. 123(10), 2354–2361 (2008)
Bartsch, H.H., et al.: Intralesional application of recombinant human tumor necrosis factor alpha induces local tumor regression in patients with advanced malignancies. Eur. J. Cancer Clin. Oncol. 25(2), 287–291 (1989)
Kwong, B., et al.: Localized immunotherapy via liposome-anchored anti-CD137+IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer Res. 73(5), 1547–1558 (2013)
Robbins, P.F., et al.: A pilot trial using lymphocytes genetically engineered with an NY-ESO-1–reactive T-cell receptor: long-term follow-up and correlates with response. Clin. Cancer Res. 21(5), 1019–1027 (2015)
Bobisse, S., et al.: Sensitive and frequent identification of high avidity neo-epitope specific CD8+ T cells in immunotherapy-naive ovarian cancer. Nat. Commun. 9(1), 1092 (2018)
Tran, E., et al.: Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 344(6184), 641–645 (2014)
Maude, S.L., et al.: Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378(5), 439–448 (2018)
Neelapu, S.S., et al.: Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377(26), 2531–2544 (2017)
Schuster, S.J., et al.: Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 377(26), 2545–2554 (2017)
Hollyman, D., et al.: Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J. Immunother. 32(2), 169 (2009)
Huang, X., et al.: Sleeping beauty transposon-mediated engineering of human primary T cells for therapy of CD19+ lymphoid malignancies. Mol. Ther. 16(3), 580–589 (2008)
Perica, K., et al.: Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano. 8(3), 2252–2260 (2014)
Perica, K., et al.: Enrichment and expansion with nanoscale artificial antigen presenting cells for adoptive immunotherapy. ACS Nano. 9(7), 6861–6871 (2015)
Zheng, B.B., et al.: Bacterium-Mimicking Vector with Enhanced Adjuvanticity for Cancer Immunotherapy and Minimized Toxicity. Adv. Funct. Mater. 29, 33 (2019)
Badie, B., Berlin, J.M.: The future of CpG immunotherapy in cancer. Immunotherapy. 5(1), 1–3 (2013)
Lin, A.Y., et al.: Gold Nanoparticle Delivery of Modified CpG Stimulates Macrophages and Inhibits Tumor Growth for Enhanced Immunotherapy. PLoS One. 8, 5 (2013)
Paciotti, G.F., et al.: Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 11(3), 169–183 (2004)
Goel, R., et al.: Biodistribution of TNF-alpha-coated gold nanoparticles in an in vivo model system. Nanomedicine. 4(4), 401–410 (2009)
Shenoi, M.M., et al.: Nanoparticle preconditioning for enhanced thermal therapies in cancer. Nanomedicine. 6(3), 545–563 (2011)
Lin, A.Y., et al.: High-density sub-100-nm peptide-gold nanoparticle complexes improve vaccine presentation by dendritic cells in vitro. Nanoscale Res. Lett. 8 (2013)
Lee, I.H., et al.: Imageable antigen-presenting gold nanoparticle vaccines for effective cancer immunotherapy in vivo. Angew. Chem. Int. Ed. Engl. 51(35), 8800–8805 (2012)
Arnáiz, B., et al.: Cellular uptake of gold nanoparticles bearing HIV gp120 oligomannosides. Bioconjug. Chem. 23(4), 814–825 (2012)
Liu, H., et al.: Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 507(7493), 519 (2014)
Kuai, R., et al.: Designer vaccine nanodiscs for personalized cancer immunotherapy. Nature Materials. 16(4), 489 (2017)
Kranz, L.M., et al.: Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 534(7607), 396 (2016)
Ramishetti, S., Peer, D.: Engineering lymphocytes with RNAi. Adv. Drug Deliv. Rev. 141, 55–66 (2019)
Falk, M.H., Issels, R.D.: Hyperthermia in oncology. Int. J. Hyperth. 17(1), 1–18 (2001)
Owusu, R.A., Abern, M.R., Inman, B.A.: Hyperthermia as adjunct to intravesical chemotherapy for bladder cancer. Biomed. Res. Int., 7 (2013)
Hildebrandt, B., et al.: The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 43(1), 33–56 (2002)
Frey, B., et al.: Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperth. 28(6), 528–542 (2012)
Schildkopf, P., et al.: Biological rationales and clinical applications of temperature controlled hyperthermia—implications for multimodal cancer treatments. Curr. Med. Chem. 17(27), 3045–3057 (2010)
Wust, P., et al.: Hyperthermia in combined treatment of cancer. Lancet Oncol. 3(8), 487–497 (2002)
Loo, C., et al.: Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol. Cancer Res. Treat. 3(1), 33–40 (2004)
Pandita, T.K., Pandita, S., Bhaumik, S.R.: Molecular parameters of hyperthermia for radiosensitization. Crit. Rev. Eukaryot. Gene Expr. 19(3), 235–251 (2009)
Takada, T., et al.: Growth inhibition of Re-Challenge B16 melanoma transplant by conjugates of melanogenesis substrate and magnetite nanoparticles as the basis for developing melanoma-targeted chemo-thermo-immunotherapy. J. Biomed. Biotechnol., 13 (2009)
Koning, G.A., et al.: Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm. Res. 27(8), 1750–1754 (2010)
Wang, C., et al.: Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 26(48), 8154–8162 (2014)
Turkevich, J., Stevenson, P.C., Hillier, J.: A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 11, 55–75 (1951)
Turkevich, J., Garton, G., Stevenson, P.: The color of colloidal gold. J. Colloid Sci. 9, 26–35 (1954)
Frens, G.: Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 241(105), 20 (1973)
Khoury, C.G., Vo-Dinh, T.: Gold nanostars for surface-enhanced Raman scattering: synthesis, characterization and optimization. J. Phys. Chem. C. 112(48), 18849–18859 (2008)
Yuan, H., et al.: Gold nanostars: surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology. 23, 1361–6528 (2012) (Electronic)
Liu, Y., et al.: A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics. 5(9), 946–960 (2015)
Yuan, H., et al.: In vivo particle tracking and photothermal ablation using plasmon-resonant gold nanostars. Nanomedicine. 8(8), 1355–1363 (2012)
Yuan, H., Fales, A.M., Vo-Dinh, T.: TAT peptide-functionalized gold Nanostars: enhanced intracellular delivery and efficient NIR photothermal therapy using ultralow irradiance. J. Am. Chem. Soc. 134(28), 11358–11361 (2012)
Abadeer, N.S., Murphy, C.J.: Recent Progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C. 120(9), 4691–4716 (2016)
Villiers, C.L., et al.: Analysis of the toxicity of gold nano particles on the immune system: effect on dendritic cell functions. J. Nanopart. Res. 12(1), 55–60 (2010)
Yen, H.J., Hsu, S.H., Tsai, C.L.: Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small. 5(13), 1553–1561 (2009)
Tsai, C.-Y., et al.: Size-dependent attenuation of TLR9 signaling by gold nanoparticles in macrophages. J. Immunol. 188(1), 68–76 (2012)
Sumbayev, V.V., et al.: Gold nanoparticles downregulate interleukin-1β-induced pro-inflammatory responses. Small. 9(3), 472–477 (2013)
Nguyen, H.T., et al.: Activation of inflammasomes by tumor cell death mediated by gold nanoshells. Biomaterials. 33(7), 2197–2205 (2012)
Visaria, R.K., et al.: Enhancement of tumor thermal therapy using gold nanoparticle–assisted tumor necrosis factor-α delivery. Mol. Cancer Ther. 5(4), 1014–1020 (2006)
Bear, A.S., et al.: Elimination of metastatic melanoma using gold nanoshell-enabled photothermal therapy and adoptive T cell transfer. PLoS One. 8(7), e69073 (2013)
Yavuz, M.S., et al.: Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 8(12), 935 (2009)
You, J., et al.: Photothermal-chemotherapy with doxorubicin-loaded hollow gold nanospheres: a platform for near-infrared light-trigged drug release. J. Control. Release. 158(2), 319–328 (2012)
Casares, N., et al.: Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 202(12), 1691–1701 (2005)
Choi, M.-R., et al.: A cellular Trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 7(12), 3759–3765 (2007)
Kennedy, L.C., et al.: T cells enhance gold nanoparticle delivery to tumors in vivo. Nanoscale Res. Lett. 6 (2011)
Liu, Y., et al.: Synergistic Immuno Photothermal Nanotherapy (SYMPHONY) for the treatment of unresectable and metastatic cancers. Sci. Rep. 7(1), 8606 (2017)
Yu, X., et al.: Inhibiting metastasis and preventing tumor relapse by triggering host immunity with tumor-targeted photodynamic therapy using photosensitizer-loaded functional Nanographenes. ACS Nano. 11(10), 10147–10158 (2017)
Fales, A.M., Yuan, H., Vo-Dinh, T.: Silica-coated gold nanostars for combined surface-enhanced Raman scattering (SERS) detection and singlet-oxygen generation: a potential nanoplatform for theranostics. Langmuir. 27(19), 12186–12190 (2011)
Fales, A.M., Yuan, H., Vo-Dinh, T.: Cell-penetrating peptide enhanced intracellular Raman imaging and photodynamic therapy. Mol. Pharm. 10(6), 2291–2298 (2013)
Fales, A.M., Crawford, B.M., Vo-Dinh, T.: Folate receptor-targeted theranostic nanoconstruct for surface-enhanced Raman scattering imaging and photodynamic therapy. ACS Omega. 1(4), 730–735 (2016)
Yan, F., et al.: Apoferritin protein cages: a novel drug nanocarrier for photodynamic therapy. Chem. Commun. (Camb.). 38, 4579–4581 (2008)
Yan, F., et al.: Cellular uptake and photodynamic activity of protein nanocages containing methylene blue photosensitizing drug. Photochem. Photobiol. 86(3), 662–666 (2010)
Wang, C., Cheng, L., Liu, Z.: Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics. 3(5), 317–330 (2013)
Wang, C., et al.: Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials. 32(26), 6145–6154 (2011)
Xu, J., et al.: Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano. 11(5), 4463–4474 (2017)
Jin, X., et al.: Aptamer-functionalized upconverting nanoformulations for light-switching cancer-specific recognition and in situ photodynamic–chemo sequential theranostics. ACS Appl. Mater. Interfaces. (2020)
Yang, Y., et al.: G-Quadruplex-based nanoscale coordination polymers to modulate tumor hypoxia and achieve nuclear-targeted drug delivery for enhanced photodynamic therapy. Nano Lett. 18(11), 6867–6875 (2018)
Lasic, D.D.: The mechanism of vesicle formation. Biochem. J. 256(1), 1–11 (1988)
Barenholz, Y.: Doxil (R)—the first FDA-approved nano-drug: lessons learned. J. Control. Release. 160(2), 117–134 (2012)
Gregoriadis, G., Saffie, R., deSouza, J.B.: Liposome-mediated DNA vaccination. FEBS Lett. 402(2–3), 107–110 (1997)
McNamara, K.P., Rosenzweig, Z.: Dye-encapsulating liposomes as fluorescence-based oxygen nanosensors. Anal. Chem. 70(22), 4853–4859 (1998)
Miranda, D., et al.: Highly-soluble cyanine J-aggregates entrapped by liposomes for in vivo optical imaging around 930 nm. Theranostics. 9(2), 381–390 (2019)
Yoon, H.J., et al.: Liposomal indocyanine green for enhanced photothermal therapy. ACS Appl. Mater. Interfaces. 9(7), 5683–5691 (2017)
Cheng, L., et al.: Renal-Clearable PEGylated Porphyrin Nanoparticles for Image-guided Photodynamic Cancer Therapy. Adv. Funct. Mater. 27, 34 (2017)
Song, X.J., et al.: Liposomes co-loaded with metformin and chlorin e6 modulate tumor hypoxia during enhanced photodynamic therapy. Nano Res. 10(4), 1200–1212 (2017)
Feng, L.Z., et al.: Theranostic liposomes with HypoxiaActivated prodrug to effectively destruct hypoxic tumors post-photodynamic therapy. ACS Nano. 11(1), 927–937 (2017)
Korbelik, M., et al.: N-dihydrogalactochitosan as immune and direct antitumor agent amplifying the effects of photodynamic therapy and photodynamic therapy-generated vaccines. Int. Immunopharmacol. 75 (2019)
DeVita, V.T., Chu, E.: A history of cancer chemotherapy. Cancer Res. 68(21), 8643–8653 (2008)
Mead, G.M., Jacobs, C.: Changing-role of chemotherapy in treatment of head and neck-cancer. Am. J. Med. 73(4), 582–595 (1982)
Petrelli, A., Giordano, S.: From single- to multi-target drugs in cancer therapy: when aspecificity becomes an advantage. Curr. Med. Chem. 15(5), 422–432 (2008)
Krysko, D.V., et al.: Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer. 12(12), 860–875 (2012)
Kroemer, G., et al.: Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31(1), 51–72 (2013)
Rios-Doria, J., et al.: Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia. 17(8), 661–670 (2015)
Ahmadzada, T., Reid, G., McKenzie, D.R.: Fundamentals of siRNA and miRNA therapeutics and a review of targeted nanoparticle delivery systems in breast cancer. Biophys. Rev. 10(1), 69–86 (2018)
Kim, H.S., Seo, H.K.: Immune checkpoint inhibitors for urothelial carcinoma. Investig Clin Urol. 59(5), 285–296 (2018)
Greish, K.: Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Cancer Nanotechnol Methods Protocols. 624, 25–37 (2010)
Kobayashi, K., et al.: Surface engineering of nanoparticles for therapeutic applications. Polym. J. 46(8), 460–468 (2014)
Zhao, X., et al.: Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials. 102, 187–197 (2016)
Zhong, Y.N., et al.: cRGD-directed, NIR-responsive and robust AuNR/PEG-PCL hybrid nanoparticles for targeted chemotherapy of glioblastoma in vivo. J. Control. Release. 195, 63–71 (2014)
Zhong, Y., et al.: Gold nanorod-cored biodegradable micelles as a robust and remotely controllable doxorubicin release system for potent inhibition of drug-sensitive and -resistant cancer cells. Biomacromolecules. 14(7), 2411–2419 (2013)
Cai, Z., et al.: NIR-triggered chemo-photothermal therapy by thermosensitive gold Nanostar@mesoporous silica@liposome-composited drug delivery systems. ACS Appl. Bio Mater. 3(8), 5322–5330 (2020)
Su, G., et al.: Mesoporous silica-coated gold nanostars with drug payload for combined chemo-photothermal cancer therapy. J. Drug Target. 27(2), 201–210 (2019)
Bisker, G., et al.: Controlled release of rituximab from gold nanoparticles for phototherapy of malignant cells. J. Control. Release. 162(2), 303–309 (2012)
Jimbow, K., et al.: Melanoma-targeted chemothermotherapy and in situ peptide immunotherapy through HSP production by using melanogenesis substrate, NPrCAP, and magnetite nanoparticles. J. Skin Cancer. 2013, 742925–742925 (2013)
Sato, A., et al.: Melanoma-targeted chemo-thermo-immuno (CTI)-therapy using N-propionyl-4-S-cysteaminylphenol-magnetite nanoparticles elicits CTL response via heat shock protein-peptide complex release. Cancer Sci. 101(9), 1939–1946 (2010)
Yamamoto, S., et al.: Three-dimensional magnetic cell array for evaluation of anti-proliferative effects of chemo-thermo treatment on cancer spheroids. Biotechnol. Bioprocess Eng. 20(3), 488–497 (2015)
Feng, L.Z., et al.: Smart pH-responsive nanocarriers based on Nano-graphene oxide for combined chemo- and photothermal therapy overcoming drug resistance. Adv. Healthc. Mater. 3(8), 1261–1271 (2014)
Zhou, F., et al.: Photo-activated chemo-immunotherapy for metastatic cancer using a synergistic graphene nanosystem. Biomaterials. 265, 120421 (2021)
Sun, R., et al.: Photoactivated H2 nanogenerator for enhanced chemotherapy of bladder cancer. ACS Nano. 14(7), 8135–8148 (2020)
Chattopadhyay, S., et al.: Synthetic immunogenic cell death mediated by intracellular delivery of STING agonist Nanoshells enhances anticancer chemo-immunotherapy. Nano Lett. 20(4), 2246–2256 (2020)
Liu, J.J., et al.: pH-Sensitive dissociable nanoscale coordination polymers with drug loading for synergistically enhanced chemoradiotherapy (vol 27, 1703832, 2017). Adv. Funct. Mater. 29, 51 (2019)
Sessa, G., Weissmann, G.: Phospholipid spherules (liposomes) as a model for biological membranes. J. Lipid Res. 9(3), 310 (1968)
Yoon, H.-J., et al.: Photothermally amplified therapeutic liposomes for effective combination treatment of cancer. ACS Appl. Mater. Interfaces. 10(7), 6118–6123 (2018)
Shen, F.Y., et al.: Oxaliplatin-/NLG919 prodrugs-constructed liposomes for effective chemoimmunotherapy of colorectal cancer. Biomaterials. 255 (2020)
Lu, J.Q., et al.: Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. ACS Nano. 12(11), 11041–11061 (2018)
Kuai, R., et al.: Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv. 4(4), eaao1736 (2018)
Seth, A., Heo, M.B., Lim, Y.T.: Poly (γ-glutamic acid) based combination of water-insoluble paclitaxel and TLR7 agonist for chemo-immunotherapy. Biomaterials. 35(27), 7992–8001 (2014)
Tian, L., et al.: Coordination Polymers Integrating Metalloimmunology with Immune Modulation to Elicit Robust Cancer Chemoimmunotherapy. CCS Chemistry.
Lu, J., et al.: Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat. Commun. 8(1), 1811 (2017)
Jemal, A., et al. Global cancer statistics. (1542–4863 (Electronic))
Zhang, L., et al.: Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Therapeut. 83(5), 761–769 (2008)
Kamyshny, A., Magdassi, S.: Conductive nanomaterials for printed electronics. Small. 10(17), 3515–3535 (2014)
Dastjerdi, R., Montazer, M.: A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids Surfaces B-Biointerfaces. 79(1), 5–18 (2010)
Vance, M.E., Kuiken, T., Vejerano, E. P., McGinnis, S.P., Hochella, M.F., Jr., Rejeski, D. and Hull, M.S., Nanotechnology in the Real World: Redeveloping the Nanomaterial Consumer Products Inventory. (2019)
Kreyling, W.G., Semmler-Behnke, M., Chaudhry, Q.: A complementary definition of nanomaterial. Nano Today. 5(3), 165–168 (2010)
Smith, A.M., Mancini, M.C., Nie, S.M.: BIOIMAGING second window for in vivo imaging. Nat. Nanotechnol. 4(11), 710–711 (2009)
Acknowledgements
This work was supported by National Institutes of Health (1R01EB028078-01A1).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cupil-Garcia, V., Crawford, B.M., Vo-Dinh, T. (2021). Nanoparticle Systems Applied for Immunotherapy in Various Treatment Modalities. In: Vo-Dinh, T. (eds) Nanoparticle-Mediated Immunotherapy. Bioanalysis, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-030-78338-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-78338-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-78337-2
Online ISBN: 978-3-030-78338-9
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)