Abstract
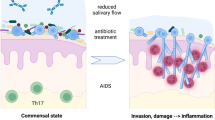
Candida albicans is an opportunistic pathogen colonizing the oropharyngeal, esophageal, and gastrointestinal mucosa in most healthy humans. In immunocompromised hosts, this fungal organism can cause mucosal candidiasis in these sites. C. albicans also causes fungemia, a serious consequence of cancer cytotoxic chemotherapy, which is thought to develop from fungal translocation through compromised mucosal barriers. Changes in endogenous bacterial population size or composition as well as changes in the host environment can transform fungal commensals into opportunistic pathogens in the upper and lower GI tract. Pioneering studies from our group have shown that a ubiquitous oral commensal of the mitis streptococcal group (Streptococcus oralis) has a mutualistic relationship with C. albicans, with C. albicans enabling streptococcal biofilm growth at mucosal sites, and S. oralis facilitating invasion of the oral and esophageal mucosa by C. albicans. In these studies, we used a cortisone-induced immunosuppression mouse model. More recently, the development of a novel mouse chemotherapy model has allowed us to examine the interactions of C. albicans with the endogenous bacterial microbiota in the oral and small intestinal mucosa, two sites adversely affected by cytotoxic chemotherapy. In this model, oral inoculation with C. albicans causes severe dysbiosis in the mucosal bacterial composition in both sites. We also found that antibiotic treatment ameliorates invasion of the oral mucosa but aggravates dissemination through the intestinal mucosa. In this chapter, we discuss work from our laboratory and others examining the relationships of C. albicans with oral bacteria and their role in mucosal homeostasis or disease.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abusleme, L., Diaz, P. I., Freeman, A. F., Greenwell-Wild, T., Brenchley, L., Desai, J. V., Ng, W. I., Holland, S. M., Lionakis, M. S., Segre, J. A., Kong, H. H., & Moutsopoulos, N. M. (2018). Human defects in STAT3 promote oral mucosal fungal and bacterial dysbiosis. JCI Insight, 3(17), 122061.
Bertolini, M., Sobue, T., Thompson, A., & Dongari-Bagtzoglou, A. (2017). Chemotherapy induces oral mucositis in mice without additional noxious stimuli. Translational Oncology, 10(4), 612–620.
Bertolini, M., Ranjan, A., Thompson, A., Diaz, P., Sobue, T., Maas, K., & Dongari-Bagtzoglou, A. (2019). Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathogens, 15(4), e1007717.
Bohm, L., Torsin, S., Tint, S. H., Eckstein, M. T., Ludwig, T., & Perez, J. C. (2017). The yeast form of the fungus Candida albicans promotes persistence in the gut of gnotobiotic mice. PLoS Pathogens, 13(10), e1006699.
Boktour, M. R., Kontoyiannis, D. P., Hanna, H. A., Hachem, R. Y., Girgawy, E., Bodey, G. P., & Raad, I. I. (2004). Multiple-species candidemia in patients with cancer. Cancer, 101, 1860–1865.
Brent, N. B. (2001). Thrush in the breastfeeding dyad: Results of a survey on diagnosis and treatment. Clinical Pediatrics, 40(9), 503–506.
Cho, I., & Blaser, M. J. (2012). The human microbiome: At the interface of health and disease. Nature Reviews Genetics, 13(4), 260–270.
Clancy, C. J., Cheng, S., & Nguyen, M. H. (2009). Animal models of candidiasis. Methods in Molecular Biology, 499, 65–76.
Clemons, K. V., Gonzalez, G. M., Singh, G., Imai, J., Espiritu, M., Parmar, R., & Stevens, D. A. (2006). Development of an orogastrointestinal mucosal model of candidiasis with dissemination to visceral organs. Antimicrobial Agents and Chemotherapy, 50(8), 2650–2657.
Cole, G. T., Halawa, A. A., & Anaissie, E. J. (1996). The role of the gastrointestinal tract in hematogenous candidiasis: From the laboratory to the bedside. Clinical Infectious Diseases, 22(Suppl. 2), S73–S88.
Cruz, M. R., Graham, C. E., Gagliano, B. C., Lorenz, M. C., & Garsin, D. A. (2013). Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infection and Immunity, 81(1), 189–200.
Dutzan, N., Abusleme, L., Bridgeman, H., Greenwell-Wild, T., Zangerle-Murray, T., Fife, M. E., Bouladoux, N., Linley, H., Brenchley, L., Wemyss, K., Calderon, G., Hong, B. Y., Break, T. J., Bowdish, D. M. E., Lionakis, M. S., Jones, S. A., Trinchieri, G., Diaz, P. I., Belkaid, Y., Konkel, J. E., & Moutsopoulos, N. M. (2017). On-going mechanical damage from mastication drives homeostatic Th17 cell responses at the oral barrier. Immunity, 46(1), 133–147.
Fan, D., Coughlin, L. A., Neubauer, M. M., Kim, J., Kim, M. S., Zhan, X., Simms-Waldrip, T. R., Xie, Y., Hooper, L. V., & Koh, A. Y. (2015). Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nature Medicine, 21(7), 808–814.
Ghannoum, M. A., Jurevic, R. J., Mukherjee, P. K., Cui, F., Sikaroodi, M., Naqvi, A., & Gillevet, P. M. (2010). Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathogens, 6(1), e1000713.
Gligorov, J., Bastit, L., Gervais, H., Henni, M., Kahila, W., Lepille, D., Luporsi, E., Sasso, G., Varette, C., Azria, D., & Candidoscope Study, G. (2011). Prevalence and treatment management of oropharyngeal candidiasis in cancer patients: Results of the French CANDIDOSCOPE study. International Journal of Radiation Oncology, Biology, Physics, 80(2), 532–539.
Gonçalves, L. S., Soares Ferreira, S. M., Souza, C. O., Souto, R., & Colombo, A. P. (2007). Clinical and microbiological profiles of human immunodeficiency virus (HIV)-seropositive Brazilians undergoing highly active antiretroviral therapy and HIV-seronegative Brazilians with chronic periodontitis. Journal of Periodontology, 78, 87.
Gonçalves, L. S., Souto, R., & Colombo, A. P. (2009). Detection of Helicobacter pylori, Enterococcus faecalis, and Pseudomonas aeruginosa in the subgingival biofilm of HIV-infected subjects undergoing HAART with chronic periodontitis. European Journal of Clinical Microbiology & Infectious Diseases, 28(11), 1335–1342.
Gow, N. A., van de Veerdonk, F. L., Brown, A. J., & Netea, M. G. (2011). Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nature Reviews Microbiology, 10(2), 112–122.
Graham, C. E., Cruz, M. R., Garsin, D. A., & Lorenz, M. C. (2017). Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proceedings of the National Academy of Sciences of the United States of America, 114(17), 4507–4512.
Hata, K., Horii, T., Miyazaki, M., Watanabe, N. A., Okubo, M., Sonoda, J., Nakamoto, K., Tanaka, K., Shirotori, S., Murai, N., Inoue, S., Matsukura, M., Abe, S., Yoshimatsu, K., & Asada, M. (2011). Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrobial Agents and Chemotherapy, 55(10), 4543–4551.
Holler, E., Butzhammer, P., Schmid, K., Hundsrucker, C., Koestler, J., Peter, K., Zhu, W., Sporrer, D., Hehlgans, T., Kreutz, M., et al. (2014). Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: Loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biology of Blood and Marrow Transplantation, 20, 640–645.
Iliev, I. D., Funari, V. A., Taylor, K. D., Nguyen, Q., Reyes, C. N., Strom, S. P., Brown, J., Becker, C. A., Fleshner, P. R., Dubinsky, M., Rotter, J. I., Wang, H. L., McGovern, D. P., Brown, G. D., & Underhill, D. M. (2012). Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science, 336(6086), 1314–1317.
Jarvis, W. R. (1995). Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clinical Infectious Diseases, 20(6), 1526–1530.
Kim, Y., & Mylonakis, E. (2011). Killing of Candida albicans filaments by Salmonella enterica serovar Typhimurium is mediated by sopB effectors, parts of a type III secretion system. Eukaryotic Cell, 10(6), 782–790.
Kleinegger, C. L., Lockhart, S. R., Vargas, K., & Soll, D. R. (1996). Frequency, intensity, species, and strains of oral Candida vary as a function of host age. Journal of Clinical Microbiology, 34(9), 2246–2254.
Koh, A. Y. (2013). Murine models of Candida gastrointestinal colonization and dissemination. Eukaryotic Cell, 12(11), 1416–1422.
Komiyama, E. Y., Lepesqueur, L. S., Yassuda, C. G., Samaranayake, L. P., Parahitiyawa, N. B., Balducci, I., & Koga-Ito, C. Y. (2016). Enterococcus species in the oral cavity: Prevalence, virulence factors and antimicrobial susceptibility. PLoS One, 11(9), e0163001.
Kong, E. F., Kucharíková, S., Van Dijck, P., Peters, B. M., Shirtliff, M. E., & Jabra-Rizk, M. A. (2015). Clinical implications of oral candidiasis: Host tissue damage and disseminated bacterial disease. Infection and Immunity, 83(2), 604–613.
Kraneveld, E. A., Buijs, M. J., Bonder, M. J., Visser, M., Keijser, B. J., Crielaard, W., & Zaura, E. (2012). The relation between oral Candida load and bacterial microbiome profiles in Dutch older adults. PLoS One, 7(8), e42770.
Krause, R., Schwab, E., Bachhiesl, D., Daxbock, F., Wenisch, C., Krejs, G. J., & Reisinger, E. C. (2001). Role of Candida in antibiotic-associated diarrhea. The Journal of Infectious Diseases, 184(8), 1065–1069.
Lagkouvardos, I., Joseph, D., Kapfhammer, M., Giritli, S., Horn, M., Haller, D., & Clavel, T. (2016). IMNGS: A comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Scientific Reports, 6, 33721.
Lalla, R. V., Latortue, M. C., Hong, C. H., Ariyawardana, A., D’Amato-Palumbo, S., Fischer, D. J., Martof, A., Nicolatou-Galitis, O., Patton, L. L., Elting, L. S., Spijkervet, F. K., Brennan, M. T., & Fungal Infections Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). (2010). A systematic review of oral fungal infections in patients receiving cancer therapy. Supportive Care in Cancer, 18(8), 985–992.
Leendertse, M., Willems, R. J., Giebelen, I. A., Roelofs, J. J., Bonten, M. J., & van der Poll, T. (2009). Neutrophils are essential for rapid clearance of Enterococcus faecium in mice. Infection and Immunity, 77(1), 485–491.
Lucatorto, F. M., Franker, C., Hardy, W. D., & Chafey, S. (1991). Treatment of refractory oral candidiasis with fluconazole. A case report. Oral Surgery, Oral Medicine, and Oral Pathology, 71(1), 42–44.
Maeda, Y., Elborn, J. S., Parkins, M. D., Reihill, J., Goldsmith, C. E., Coulter, W. A., Mason, C., Millar, B. C., Dooley, J. S., Lowery, C. J., Ennis, M., Rendall, J. C., & Moore, J. E. (2011). Population structure and characterization of viridans group streptococci (VGS) including Streptococcus pneumoniae isolated from adult patients with cystic fibrosis (CF). Journal of Cystic Fibrosis, 10(2), 133–139.
Mason, K. L., Erb Downward, J. R., Falkowski, N. R., Young, V. B., Kao, J. Y., & Huffnagle, G. B. (2012a). Interplay between the gastric bacterial microbiota and Candida albicans during postantibiotic recolonization and gastritis. Infection and Immunity, 80(1), 150–158.
Mason, K. L., Erb Downward, J. R., Mason, K. D., Falkowski, N. R., Eaton, K. A., Kao, J. Y., Young, V. B., & Huffnagle, G. B. (2012b). Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infection and Immunity, 80(10), 3371–3380.
Meunier, F., Gerard, M., Richard, V., Debusscher, L., Bleiberg, H., & Malengrau, A. (1989). Hepatic candidosis in a patient with acute leukemia. Mycoses, 32(8), 421–426.
Moran, C., Grussemeyer, C. A., Spalding, J. R., Benjamin, D. K., Jr., & Reed, S. D. (2010). Comparison of costs, length of stay, and mortality associated with Candida glabrata and Candida albicans bloodstream infections. American Journal of Infection Control, 38(1), 78–80.
Nagy, K. N., Sonkodi, I., Szoke, I., Nagy, E., & Newman, H. N. (1998). The microflora associated with human oral carcinomas. Oral Oncology, 34(4), 304–308.
Nicolatou-Galitis, O., Dardoufas, K., Markoulatos, P., Sotiropoulou-Lontou, A., Kyprianou, K., Kolitsi, G., Pissakas, G., Skarleas, C., Kouloulias, V., Papanicolaou, V., Legakis, N. J., & Velegraki, A. (2001). Oral pseudomembranous candidiasis, herpes simplex virus-1 infection, and oral mucositis in head and neck cancer patients receiving radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF) mouthwash. Journal of Oral Pathology & Medicine, 30(8), 471–480.
Olczak-Kowalczyk, D., Daszkiewicz, M., Krasuska-Sławińska, Dembowska-Bagińska, B., Gozdowski, D., Daszkiewicz, P., Fronc, B., & Semczuk, K. (2012). Bacteria and Candida yeasts in inflammations of the oral mucosa in children with secondary immunodeficiency. Journal of Oral Pathology & Medicine, 41(7), 568–576.
Passalacqua, G., Albano, M., Canonica, G. W., Bachert, C., Van Cauwenberge, P., Davies, R. J., Durham, S. R., Kontou-Fili, K., Horak, F., & Malling, H. J. (2000). Inhaled and nasal corticosteroids: Safety aspects. Allergy, 55(1), 16–33.
Pei, Z., Bini, E. J., Yang, L., Zhou, M., Francois, F., & Blaser, M. J. (2004). Bacterial biota in the human distal esophagus. Proceedings of the National Academy of Sciences of the United States of America, 101(12), 4250–4255.
Perlroth, J., Choi, B., & Spellberg, B. (2007). Nosocomial fungal infections: Epidemiology, diagnosis, and treatment. Medical Mycology, 45(4), 321–346.
Peters, B. M., & Noverr, M. C. (2013). Candida albicans-Staphylococcus aureus polymicrobial peritonitis modulates host innate immunity. Infection and Immunity, 81(6), 2178–2189.
Polak-Wyss A. Protective effect of human granulocyte colony stimulating factor (hG-CSF) on Candida infections in normal and immunosuppressed mice. Mycoses. 1991;34(3–4):109–18. PubMed PMID: 1721105.
Ponnuvel, K. M., Rajkumar, R., Menon, T., & Sankaranarayanan, V. S. (1996). Role of Candida in indirect pathogenesis of antibiotic associated diarrhoea in infants. Mycopathologia, 135(3), 145–147.
Puig-Asensio, M., Ruiz-Camps, I., Fernandez-Ruiz, M., Aguado, J. M., Munoz, P., Valerio, M., Delgado-Iribarren, A., Merino, P., Bereciartua, E., Fortun, J., et al. (2015). Epidemiology and outcome of candidaemia in patients with oncological and haematological malignancies: Results from a population-based surveillance in Spain. Clinical Microbiology and Infection, 21, 491–495.
Redding, S. W., Kirkpatrick, W. R., Coco, B. J., Sadkowski, L., Fothergill, A. W., Rinaldi, M. G., Eng, T. Y., & Patterson, T. F. (2002). Candida glabrata oropharyngeal candidiasis in patients receiving radiation treatment for head and neck cancer. Journal of Clinical Microbiology, 40(5), 1879–1881.
Russell, C., & Lay, K. M. (1973). Natural history of Candida species and yeasts in the oral cavities of infants. Archives of Oral Biology, 18(8), 957–962.
Sawyer, R. T., & Harmsen, A. G. (1989). The relative contribution of resident pulmonary alveolar macrophage and inflammatory polymorphonuclear neutrophils in host resistance to pulmonary infection by Candida albicans. Mycopathologia, 108(2), 95–105.
Shankar, J., Solis, N. V., Mounaud, S., Szpakowski, S., Liu, H., Losada, L., Nierman, W. C., & Filler, S. G. (2015). Using Bayesian modelling to investigate factors governing antibiotic-induced Candida albicans colonization of the GI tract. Scientific Reports, 5, 8131.
Sjovall, J., Huitfeldt, B., Magni, L., & Nord, C. E. (1986). Effect of beta-lactam prodrugs on human intestinal microflora. Scandinavian Journal of Infectious Diseases Supplementum, 49, 73–84.
Sobue, T., Diaz, P., Xu, H., Bertolini, M., & Dongari-Bagtzoglou, A. (2016). Experimental models of C. albicans-Streptococcal Co-infection. Methods in Molecular Biology, 1356, 137–152.
Sobue, T., Bertolini, M., Thompson, A., Peterson, D. E., Diaz, P. I., & Dongari-Bagtzoglou, A. (2018). Chemotherapy-induced oral mucositis and associated infections in a novel organotypic model. Molecular Oral Microbiology, 33(3), 212–223.
Solis, N. V., & Filler, S. G. (2012). Mouse model of oropharyngeal candidiasis. Nature Protocols, 7(4), 637–642.
Sparo, M., Delpech, G., & García Allende, N. (2018). Impact on public health of the spread of high-level resistance to gentamicin and vancomycin in enterococci. Frontiers in Microbiology, 9, 3073.
St Leger, A. J., Desai, J. V., Drummond, R. A., Kugadas, A., Almaghrabi, F., Silver, P., Raychaudhuri, K., Gadjeva, M., Iwakura, Y., Lionakis, M. S., & Caspi, R. R. (2017). An ocular commensal protects against corneal infection by driving an interleukin-17 response from mucosal γδ T cells. Immunity, 47(1), 148–158.e5.
Swidsinski, A., Weber, J., Loening-Baucke, V., Hale, L. P., & Lochs, H. (2005). Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. Journal of Clinical Microbiology, 43(7), 3380–3389.
Tampakakis, E., Peleg, A. Y., & Mylonakis, E. (2009). Interaction of Candida albicans with an intestinal pathogen, Salmonella enterica serovar Typhimurium. Eukaryotic Cell, 8(5), 732–737.
Teoh, F., & Pavelka, N. (2016). How chemotherapy increases the risk of systemic candidiasis in cancer patients: Current paradigm and future directions. Pathogens, 5(1), E6.
Villafuerte, K. R. V., Martinez, C. J. H., Dantas, F. T., Carrara, H. H. A., Dos Reis, F. J. C., & Palioto, D. B. (2018). The impact of chemotherapeutic treatment on the oral microbiota of patients with cancer: A systematic review. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, 125(6), 552–566.
Wilson, L. S., Reyes, C. M., Stolpman, M., Speckman, J., Allen, K., & Beney, J. (2002). The direct cost and incidence of systemic fungal infections. Value in Health, 5(1), 26–34.
Xu, H., Sobue, T., Thompson, A., Xie, Z., Poon, K., Ricker, A., Cervantes, J., Diaz, P. I., & Dongari-Bagtzoglou, A. (2014). Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cellular Microbiology, 16(2), 214–231.
Xu, H., Sobue, T., Bertolini, M., Thompson, A., & Dongari-Bagtzoglou, A. (2016). Streptococcus oralis and Candida albicans synergistically activate mu-calpain to degrade E-cadherin from oral epithelial junctions. The Journal of Infectious Diseases, 214(6), 925–934.
Xu, H., Sobue, T., Bertolini, M., Thompson, A., Vickerman, M., Nobile, C. J., & Dongari-Bagtzoglou, A. (2017). S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence, 8(8), 1602–1617.
Yang, C. H., Chew, K. Y., Solomkin, J. S., Lin, P. Y., Chiang, Y. C., & Kuo, Y. R. (2013). Surgical site infections among high-risk patients in clean-contaminated head and neck reconstructive surgery: Concordance with preoperative oral flora. Annals of Plastic Surgery, 71(Suppl. 1), S55–S60.
Yano, J., Lilly, E., Barousse, M., & Fidel, P. L., Jr. (2010). Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infection and Immunity, 78(12), 5126–5137.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Bertolini, M., Dongari-Bagtzoglou, A. (2019). The Relationship of Candida albicans with the Oral Bacterial Microbiome in Health and Disease. In: Belibasakis, G.N., Hajishengallis, G., Bostanci, N., Curtis, M.A. (eds) Oral Mucosal Immunity and Microbiome. Advances in Experimental Medicine and Biology, vol 1197. Springer, Cham. https://doi.org/10.1007/978-3-030-28524-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-28524-1_6
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28523-4
Online ISBN: 978-3-030-28524-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)