Abstract
Glioblastoma (GBM) is the most common malignant brain tumor and accounts for 70% of all primary brain tumors in adults. Despite aggressive multimodal therapy including surgery, radiation, and chemotherapy, the prognosis remains poor with a median survival of around 2 years. Tumor-treating fields (TTFields) is a new frontier in cancer therapy and has been recently approved for the treatment of GBM. This chapter discusses emerging concepts of brain tumor management, with special emphasis toward novel therapeutic approaches. Recent neuroimaging advances including novel physiologic and metabolic neuroimaging techniques and their role in monitoring treatment-related temporal characteristics and assessing response to this unique treatment modality will also be reviewed.
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.
Similar content being viewed by others
Keywords
- Glioblastoma
- Tumor-treating fields
- Response assessment
- Diffusion-tensor imaging, Perfusion-weighted imaging
- Echo-planar spectroscopic imaging
1 Introduction
Glioblastoma (GBM) is the most malignant tumor in the brain, representing 30% of all central nervous system tumors (CNST) and 70% of primary malignant CNST. GBM is the cause of 225,000 deaths per year throughout the world. It has an incidence of 5 per 100,000 persons, affects 1.5 times more men than women, and is diagnosed at an average age of 64 [1]. The current standard of care treatment for patients with GBM includes maximal safe resection followed by radiotherapy and chemotherapy using temozolomide (TMZ). However, the prognosis of GBM is miserable with a median overall survival (OS) of only 12–18 months following diagnosis [2]. Because of continuing progress in the quest for an effective treatment, the therapeutic armamentarium for patients with GBM has grown significantly over the past decade. However, newly tested adjuvant strategies such as the addition of bevacizumab [3], modified TMZ dosing [4], or use of other targeted therapies [5, 6] have failed to significantly improve OS. These limitations necessitate the investigation of novel therapies to treat patients with GBMs.
2 Tumor-Treating Fields: Scientific Basis
Recently, the US Food and Drug Administration (FDA) approved the use of alternating electric fields, also known as tumor-treating fields (TTFields), as a novel modality for the treatment of patients with newly diagnosed and recurrent GBM. It consists of a portable, noninvasive, and in-home use battery-operated medical device involving four insulated transducer arrays composed of biocompatible ceramic discs (9 discs per array) that are applied to the shaved scalp of a patient. The position and size of the transducer arrays can be adjusted depending upon patient head size, tumor dimensions, and location. TTFields deliver oscillating electric energy at low intensity (1–3 V/cm) and at an intermediate frequency (200–300 kHz) as a loco-regional intervention. TTFields produce antimitotic effects by physically interacting with highly charged macromolecules and organelles in rapidly dividing cancer cells to disrupt their proper alignment during the metaphase and/or anaphase stages of mitotic cell division, mainly sparing the effect of oscillating electric fields on normal quiescent cells [7, 8]. In one study [9], treating U-118 glioma cells with TTFields in combination with standard chemotherapeutic drugs (Paclitaxel, Doxorubicin, Cyclophosphamide) resulted in the destruction of most living cells after 70 hours of treatment, while the drugs or TTFields alone only slowed down cancer cell proliferation, suggesting that TTFields should be combined with another treatment modalities to reach optimal effectiveness. In another study [10], rats bearing intracranial GBM were treated with TTFields for 6 days, leading to smaller tumors compared with untreated rats. Interestingly, this study underlined the necessity of applying TTFields in several directions to yield antitumor efficacy. There also appears to be a time-dependent treatment effect, with optimal efficacy being observed when wearing the treatment mask at least 18 hours per day (75%) [11].
3 Tumor-Treating Fields: Clinical Application in GBM Patients
TTFields have been widely used in the treatment of a variety of cancers, for example: glioma, melanoma, and adenocarcinoma, with favorable safety profiles and without significant adverse effects in patients [8, 12]. Previously, promising findings of large-scale multinational clinical trials have also been reported in patients with GBM. In particular, in a phase III clinical study [11] involving 466 patients, the addition of TTFields to standard therapy was shown to increase median OS from 15.6 to 20.5 months (hazard ratio = 0.64, p = 0.0042). The 2-year survival rate was approximately 50% greater with TTFields plus TMZ versus TMZ alone: 43% versus 29%. Additionally, improved quality of life with better cognitive and emotional functions was observed in TTFields treated cohorts of patients [11]. Moreover, the treatment had limited adverse events, mainly restricted to mild or moderate skin irritations beneath the transducer arrays from wearing the device. Therefore, these results were exciting for both physicians and patients alike.
4 Tumor-Treating Fields: Advanced Neuroimaging Techniques
We at the University of Pennsylvania are investigating the utility of advanced neuroimaging techniques in monitoring treatment-related temporal characteristics and assessing response to this unique treatment modality. Anatomic magnetic resonance imaging (MRI) of the brain provides excellent soft tissue contrast and is routinely used for the noninvasive characterization of brain tumors. However, conventional imaging utilizing “Response Assessment in Neuro-Oncology” (RANO) criteria is usually not reliable for assessment of the treatment response in patients with GBM due to the lack of specificity [13]. Consequently, there is an urgent need to develop increasingly accurate quantitative imaging biomarkers for early evaluation of treatment response. These biomarkers are the premise of personalized treatment, enabling change or discontinuation of therapy to prevent ineffective treatment or unfavorable events. Moreover, identification of treatment failure may help reduce adverse economic consequences. This is highly relevant because the cost of TTFields therapy is considerably high at $21,000 per month [14]. Advanced MR imaging techniques such as diffusion-tensor imaging (DTI) [15], dynamic susceptibility contrast (DSC)-perfusion weighted imaging (PWI) [16, 17], and proton MR spectroscopy (1H MRS) [18, 19] have shown great potential in evaluating treatment response to different therapeutic regimens in GBM patients. DTI is an MR imaging technique used to noninvasively investigate the cyto-architectural integrity of brain structures by measuring the anisotropy of microscopic water diffusivity. Along with more commonly used DTI parameters such as mean diffusivity (MD) and fractional anisotropy (FA), geometrical DTI indices such as the coefficients of linear anisotropy (CL) and planar anisotropy (CP) can be helpful in characterizing tissue organization and orientation of white matter tracts in the brain. Relative cerebral blood volume (rCBV) derived from PWI reflects tumor angiogenesis and vascularity. 1H MRS is a method that measures metabolic markers of neoplastic activity [20]. Spectra from brain tumors have increased choline (Cho), which correlates with membrane biosynthesis by proliferating cells, and reduced N-acetylasparate (NAA), which indicates loss of neuronal integrity due to tumor cell infiltration [21]. 3D-Echo planar spectroscopic imaging (EPSI) allows acquisition of volumetric metabolite maps with high spatial resolution, minimizing partial-volume averaging effects [22, 23]. Thus, 3D-EPSI may be helpful in providing metabolite information from the entire volume of a neoplasm. The potential of 3D-EPSI has been reported in characterizing glioma grades [24], mapping glycine distribution in gliomas [25], planning radiation therapy for GBM patients [26], identifying residual tumors following radiation therapy [27], evaluating response to epigenetic modifying agents in recurrent GBM [28], in assessing the effect of whole brain radiation therapy on normal brain parenchyma in patients with metastases [29] and in distinguishing true progression (TP) from pseudoprogression (PsP) in GBM patients [30].
5 Tumor-Treating Fields: Initial Experience
We have recently reported our initial experience of assessing short-term (up to 2 months) response to TTFields in a newly diagnosed patient with left thalamic GBM using physiological and metabolic MR imaging techniques [31]. In addition to conventional imaging, the patient also underwent DTI, PWI, and 3D-EPSI on a 3 T MRI scanner prior to initiation of TTFields (baseline) and at 1- and 2-month follow-ups. The values of various advanced imaging parameters such as MD, FA, rCBV, and choline/creatine (Cho/Cr) were measured from the contrast-enhancing region of the neoplasm at each time point using a previously described method [32]. Tumor size decreased from 32.5 × 27.7 mm (baseline) to 25.8 × 24.9 mm (2nd follow up), as seen on the postcontrast T1-weighted images (Fig. 9.1). Tumor volume also steadily declined at the 1st (~12%) and 2nd (~34%) follow-up periods relative to the baseline. Representative images and parametric maps from baseline and follow-up time-points are presented in Fig. 9.2. Percent changes in volume, MD, FA, rCBVmax, and Cho/Cr from baseline to post-TTFields at 1- and 2-month follow-up periods are shown in Fig. 9.3. We also found a moderate increase in MD (~11%) along with decreases in FA (~23%) from enhancing regions of neoplasms. Previous studies [33, 34] have reported increased MD and reduced FA from the tumor in patients with gliomas treated with chemoradiation therapy . However, the interpretation of changes in MD following radiation therapy and adjuvant chemotherapy is complex because of co-localization of treatment-induced gliosis, necrosis, and edema [35]. In an earlier study from our group, Wang et al. [36] reported higher MD and significantly lower FA in post-treatment GBM patients with PsP compared with those with TP, suggesting that elevated MD and reduced FA are associated with favorable treatment response. Our DTI results are in agreement with these studies and imply that DTI can assess therapeutic response to TTFields. We believe that cellular growth inhibition and associated cell death at 2 months might have accounted for the large increase in MD observed in our patient. It has been widely reported that organized microstructures secondary to closely packed proliferating tumor cells in gliomas results in high FA [34, 37]. A 23% reduction in FA in the current case may be due to reduced cell density and incoherent orientation of neoplastic cells.
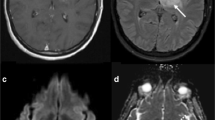
A 51-year-old patient with newly diagnosed GBM treated with TTFields plus TMZ. Axial FLAIR images at three time points demonstrate a heterogeneous mass centered in the left thalamus with surrounding signal abnormality. This mass appears hypointense on the corresponding T1-weighted images and demonstrates heterogeneous peripheral enhancement with central necrotic core on the corresponding postcontrast T1-weighted images. (Reprinted with permission from Ref. [31])
Axial co-registered contrast-enhanced T1-weighted image and corresponding MD, FA, and CBV maps are shown at baseline and at a 2-month follow-up period. (Reprinted with permission from [31])
Percentage change in parameters from baseline to 1- and 2-month follow-up periods. Trends towards decreased tumor volume, rCBVmax, Cho/Cr, and FA along with an increased MD were observed at follow-up relative to baseline indicating tumor growth arrest. (Reprinted with permission from [31])
We also observed a moderate decline in rCBVmax (6.21%) at 2 months relative to baseline. Rich capillary networks secondary to angiogenesis are a common feature of GBMs, responsible for high rCBV [38]. Several studies [16, 17, 39] have reported reduced rCBV in gliomas following radiotherapy and anti-angiogenetic therapy. Fibrinoid necrosis, endothelial injury, and occlusion of blood vessels have been proposed as potential reasons for decreased rCBV levels in treated GBMs [40]. In agreement with these studies, reduced rCBV were also noted in the present case, suggesting reduced vascularity and tissue perfusion within the tumor bed. A previous study reported substantial decrease in the levels of CD34 (an immunohistochemical marker of micro-vessel density) and downregulation of vascular endothelial growth factors (VEGF) in murine melanomas exposed to intermediate frequency alternating electric field compared to the control group [41]. While it is not clear how a combination of TTFields and TMZ chemotherapy modulates tumor vasculature of gliomas, it may be speculated that inhibited angiogenesis might have caused decreased perfusion in our case.
Several prior 1H MRS studies [18, 19, 39] have reported decreased levels of Cho as a surrogate marker of positive treatment response in patients with brain tumors. In accordance with these previous studies, we also observed decreased levels of Cho/Cr at the 2-month period following treatment (Fig. 9.4). It is well documented that Cho content correlates with cell density and with indices of cellular proliferation [42]. We believe that reduction in Cho in our case was most likely a direct consequence of the combined antiproliferative effect of TTFields and TMZ on cellular metabolism of gliomas.
Red volumes in Cho/Cr maps correspond to voxels that exceed a threshold value of 0.55 at baseline and at 2-month follow-up period. The total number of voxels that exceed the threshold value of 0.55 were 50 at baseline and 34 at 2nd follow-up, suggesting reduced levels of Cho/Cr relative to baseline. (Reprinted with permission from [31])
Taken together, our initial observations [31] indicate that a multiparametric approach utilizing the unique strengths of advanced imaging techniques as performed in the present case may provide a comprehensive assessment of treatment response. Our work is in progress and we are currently recruiting and evaluating patients with newly diagnosed, as well as recurrent GBM treated with TTFields in an ongoing clinical trial.
6 Conclusion
The identification of novel image-based biomarkers may be helpful in determining early and true therapeutic response to TTFields in patients with GBM. However, it is difficult to compare the results of individual studies because of methodological differences and varying clinical endpoints. Analytical methods of advanced MR imaging techniques can vary, including subjective/qualitative evaluation of parametric maps, user-defined region of interest values (using mean, median, maximum, or minimum), histogram analysis, and voxel-wise analysis (i.e., PRMs and fDMs). It should be noted that there is a need to establish universal quantitative imaging biomarker thresholds to evaluate treatment response to TTFields in GBMs. We believe that adequately powered, randomized, placebo-controlled, multicenter studies using optimal acquisition parameters of advanced MR imaging techniques, along with standardized postprocessing methods, are warranted to comprehensively determine the potential efficacy of TTFields in patients with GBM. This approach will enhance the decision-making process in the use of this novel treatment modality.
References
Bush, N. A., Chang, S. M., & Berger, M. S. (2017). Current and future strategies for treatment of glioma. Neurosurgical Review, 40, 1–14.
Stupp, R., Mason, W. P., van den Bent, M. J., et al. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine, 352, 987–996.
Chinot, O. L., & Reardon, D. A. (2014). The future of antiangiogenic treatment in glioblastoma. Current Opinion in Neurology, 27, 675–682.
Walker, G. V., Gilbert, M. R., Prabhu, S. S., et al. (2013). Temozolomide use in adult patients with gliosarcoma: An evolving clinical practice. Journal of Neuro-Oncology, 112, 83–89.
Batchelor, T. T., Gerstner, E. R., Emblem, K. E., et al. (2013). Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proceedings of the National Academy of Sciences of the United States of America, 110, 19059–19064.
Westphal, M., Heese, O., Steinbach, J. P., et al. (2015). A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. European Journal of Cancer, 51, 522–532.
Stupp, R., Wong, E. T., Kanner, A. A., et al. (2012). NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. European Journal of Cancer, 48(14), 2192–2202.
Pless, M., & Weinberg, U. (2011). Tumor treating fields: Concept, evidence and future. Expert Opinion on Investigational Drugs, 20(8), 1099–1106.
Kirson, E. D., Schneiderman, R. S., Dbalý, V., et al. (2009). Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Medical Physics, 8(9), 1.
Kirson, E. D., Gurvich, Z., Schneiderman, R., et al. (2004). Disruption of cancer cell replication by alternating electric fields. Cancer Research, 64(9), 3288–3295.
Stupp, R., Taillibert, S., Kanner, A. A., et al. (2015). Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. Journal of the American Medical Association, 314(23), 2535–2543.
Wong, E. T., Lok, E., & Swanson, K. D. (2015). An evidence-based review of alternating electric fields therapy for malignant gliomas. Current Treatment Options in Oncology, 16(8), 40. https://doi.org/10.1007/s11864-015-0353-5.
Jackson, E. F., Barboriak, D. P., Bidaut, L. M., & Meyer, C. R. (2009). Magnetic resonance assessment of response to therapy: tumor change measurement, truth data and error sources. Translational Oncology, 2(4), 211–215.
Bernard-Arnoux, F., Lamure, M., Ducray, F., et al. (2016). The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro-Oncology, 18, 1129–1136.
Saraswathy, S., Crawford, F. W., Lamborn, K. R., et al. (2009). Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. Journal of Neuro-Oncology, 91(1), 69–81.
Schmainda, K. M., Prah, M., Connelly, J., et al. (2014). Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro-Oncology, 16(6), 880–888.
Aquino, D., Di Stefano, A. L., Scotti, A., et al. (2014). Parametric response maps of perfusion MRI may identify recurrent glioblastomas responsive to bevacizumab and irinotecan. PLoS One, 9(3), e90535. https://doi.org/10.1371/journal.pone.0090535.
Jeon, J. Y., Kovanlikaya, I., Boockvar, J. A., et al. (2012). Metabolic response of glioblastoma to superselective intraarterial cerebral infusion of bevacizumab: A proton MR spectroscopic imaging study. American Journal of Neuroradiology, 33(11), 2095–2102.
Muruganandham, M., Clerkin, P. P., Smith, B. J., et al. (2014). 3-Dimensional magnetic resonance spectroscopic imaging at 3 Tesla for early response assessment of glioblastoma patients during external beam radiation therapy. International Journal of Radiation Oncology, Biology, Physics, 90(1), 181–189.
Chawla, S., Wang, S., Wolf, R. L., et al. (2007). Arterial spin labelling and magnetic resonance spectroscopy in differentiation of gliomas. American Journal of Neuroradiology, 28, 1683–1689.
Chawla, S., Oleaga, L., Wang, S., et al. (2010). Role of proton magnetic resonance spectroscopy in differentiating oligodendrogliomas from astrocytomas. Journal of Neuroimaging, 20, 3–8.
Ebel, A., Soher, B. J., & Maudsley, A. A. (2001). Assessment of 3D proton MR echo-planar spectroscopic imaging using automated spectral analysis. Magnetic Resonance in Medicine, 46, 1072–1078.
Maudsley, A. A., Darkazanli, A., Alger, J. R., et al. (2006). Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR in Biomedicine, 19(4), 492–503.
Roy, B., Gupta, R. K., Maudsley, A. A., et al. (2013). Utility of multiparametric 3-T MRI for glioma characterization. Neuroradiology, 55, 603–613.
Maudsley, A. A., Gupta, R. K., Stoyanova, R., et al. (2014). Mapping of glycine distributions in gliomas. American Journal of Neuroradiology, 35, S31–S36.
Parra, N. A., Maudsley, A. A., Gupta, R. K., et al. (2014). Volumetric spectroscopic imaging of glioblastoma multiforme radiation treatment volumes. International Journal of Radiation Oncology, Biology, Physics, 90, 376–384.
Lin, D., Lin, Y., Link, K., et al. (2016). Echoplanar magnetic resonance spectroscopic imaging before and following radiation therapy in patients with high-grade glioma. International Journal of Radiation Oncology, Biology, Physics, 96(2S), E133–E134.
Shim, H., Holder, C. A., & Olson, J. J. (2013). Magnetic resonance spectroscopic imaging in the era of pseudoprogression and pseudoresponse in glioblastoma patient management. CNS Oncology, 2, 393–396.
Chawla, S., Wang, S., Kim, S., et al. (2015). Radiation injury to the normal brain measured by 3D-echo-planar spectroscopic imaging and diffusion tensor imaging: Initial experience. Journal of Neuroimaging, 25, 97–104.
Verma, G., Chawla, S., Mohan, S., et al. (2019). Differentiation of true progression from pseudoprogression in patients with glioblastoma using whole brain echo-planar spectroscopic imaging. NMR in Biomedicine, 32(2), e4042. https://doi.org/10.1002/nbm.4042. Epub 2018 Dec 17.
Mohan S, Chawla S, Wang S, Verma G, Skolnik A, Brem S, Peters KB, Poptani H. (2016). Assessment of early response to tumor-treating fields in newly diagnosed glioblastoma using physiologic and metabolic MRI: initial experience. CNS Oncology, 5(3):137–144. https://doi.org/10.2217/cns-2016-0003. Epub 2016 Apr 14. PubMed PMID: 27076281; PubMed Central PMCID: PMC6042635.
Wang, S., Kim, S., Chawla, S., et al. (2009). Differentiation between glioblastomas and solitary brain metastases using diffusion tensor imaging. NeuroImage, 44(3), 653–660.
Zhang, J., van Zijl, P. C. M., Laterra, J., et al. (2007). Unique patterns of diffusion directionality in rat brain tumors revealed by high-resolution diffusion tensor MRI. Magnetic Resonance in Medicine, 58, 454–462.
Beppu, T., Inoue, T., Shibata, Y., et al. (2005). Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surgical Neurology, 63, 56–61.
Tomura, N., Narita, K., Izumi, J.-I., et al. (2006). Diffusion changes in a tumor and peritumoral tissue after stereotactic irradiation for brain tumors: Possible prediction of treatment response. Journal of Computer Assisted Tomography, 30(3), 496–500.
Wang, S., Martinez-Lage, M., Sakai, Y., et al. (2016). Differentiating tumor progression from pseudoprogression in patients with glioblastomas using diffusion tensor imaging and dynamic susceptibility contrast MRI. American Journal of Neuroradiology, 37(1), 28–36.
Kinoshita, M., Hashimoto, N., Goto, T., et al. (2008). Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. NeuroImage, 43(1), 29–35.
Lee, S. J., Kim, J. H., Kim, Y. M., et al. (2001). Perfusion MR imaging in gliomas: Comparison with histologic tumor grade. Korean Journal of Radiology, 2, 1–7.
Khan, M. N., Sharma, A. M., Pitz, M., et al. (2016). High-grade glioma management and response assessment-recent advances and current challenges. Current Oncology, 23(4), e383–e391. https://doi.org/10.3747/co.23.3082.
Liu, X. J., Duan, C. F., Fu, W. W., et al. (2015). Correlation between magnetic resonance perfusion weighted imaging of radiation brain injury and pathology. Genetics and Molecular Research, 14(4), 16317–16324.
Chen, H., Liu, R., Liu, J., & Tang, J. (2012). Growth inhibition of malignant melanoma by intermediate frequency alternating electric fields, and the underlying mechanisms. The Journal of International Medical Research, 40, 85–94.
Miller, B. L., Chang, L., Booth, R., et al. (1996). In vivo 1H MRS choline: Correlation with in vitro chemistry/histology. Life Sciences, 58, 1929–1935.
Acknowledgments
The authors would also like to thank the University of Pennsylvania radiology research team, Lisa Desiderio, Lauren Karpf, and MRI technicians, for their valuable contributions to this project.
Disclosures
This study was funded in part by a grant from Novocure Ltd., Haifa, Israel.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter
Mohan, S., Wang, S., Chawla, S. (2019). Advanced Multiparametric Imaging for Response Assessment to Tumor-Treating Fields in Patients with Glioblastoma. In: Makarov, S., Horner, M., Noetscher, G. (eds) Brain and Human Body Modeling. Springer, Cham. https://doi.org/10.1007/978-3-030-21293-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-21293-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21292-6
Online ISBN: 978-3-030-21293-3
eBook Packages: EngineeringEngineering (R0)