Abstract
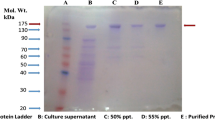
Streptococcus mutans is the major etiological agent in the development of smooth surface caries. The bacteria secrete glucosyltransferases (GTFs) that catalyze the breakdown of dietary sucrose to yield fructose for fermentative metabolism and glucose for the synthesis of glucan polymers.5 These glucans consist of α-1,3- and α-1,6-linkages and α-1,3,6-branch points to various degrees. The name mutan was given to glucans that contain a high degree of α-1,3-linkages which render the molecule water-insoluble and allow it to stick to smooth surfaces such as the teeth.3 Bacteria that lack the ability to produce glucans have been shown to be far less cariogenic than the wild-type.6 S. mutans secretes, in addition to the GTFs, a glucan binding protein (GBP) that has no known enzymatic activity. The role of GBP is postulated to be either to mediate cell-to-cell aggregation, cell-to-surface adhesion, and/or enhance the cohesiveness of plaque.1 GBP and all GTFs sequenced to date share a homologous sequence at the carboxyl terminus which is composed of a series of repeats that are thought to comprise the glucan binding domain (GBD).2 We were able to employ various fusion proteins that contain the GBD from GBP to show that this domain and its location at the C-terminus are vital to the ability of the native protein to bind to different glucans. An affinity electrophoresis assay was used to test the native GBP and two fusion proteins for their ability to bind to dextran (α-1,6-glucan), mutan (α-1,3- and α-1,6-glucan) or pseudonigeran (α-1,3-glucan). These polysaccharides were added to non-denaturing polyacrylamide gels before polymerization such that the glucan would be equally distributed within the gel matrix. Migration of proteins that bind to the polysaccharide will be greatly retarded compared to proteins that do not bind the glucans, and compared to negative controls run without any carbohydrate additions.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Banas, J. A., R. R. B. Russell, and J. J. Ferretti. 1990. Sequence analysis of the gene for the glucan binding protein of Streptococcus mutans Ingbritt. Infect. Immun. 58: 667–673
Giffard, P. M. and N. A. Jacques. 1994. Definition of a fundamental repeating unit in streptococcal glucosyltransferase glucan-binding regions and related sequences. J. Dent. Res. 73: 1133–1141
Hare, M. D., S. Svensson, and G. J. Walker. 1978. Characterization of the extracellular, water-insoluble α-D-glucans of oral streptococci by methylation analysis, and by enzymatic synthesis and degradation. Carbohydr. Res. 66: 245–264
Landale, E. C. and M. M. McCabe. 1987. Characterization by affinity electrophoresis of an α-1,6-glucan binding protein from Streptococcus sobrinus. Infect. Immun. 55: 3011–3016
Loesche, W. L. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50: 353–380
Munro, C. L., S. M. Michalek, and F. L. Macrina. 1991. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect. Immun. 59:2316–2323
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1997 Springer Science+Business Media New York
About this chapter
Cite this chapter
Haas, W., Banas, J.A. (1997). The Glucan Binding Domain of the Streptococcus mutans Glucan Binding Protein. In: Horaud, T., Bouvet, A., Leclercq, R., de Montclos, H., Sicard, M. (eds) Streptococci and the Host. Advances in Experimental Medicine and Biology, vol 418. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-1825-3_165
Download citation
DOI: https://doi.org/10.1007/978-1-4899-1825-3_165
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4899-1827-7
Online ISBN: 978-1-4899-1825-3
eBook Packages: Springer Book Archive