Abstract
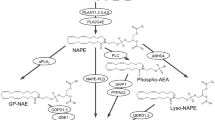
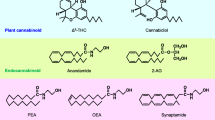
Anandamide (arachidonoyl-ethanolamide, AnNH) was the first brain endogenous substance to be proposed as a physiological agonist at central cannabinoid receptors1. Apart from its cannabimimetic properties (recently reviewed in ref. 2), a role as a neuronal chemical signal for AnNH was suggested also by the finding of molecular mechanisms for its inactivation and Ca2+-dependent biosynthesis by rat central neurons3. The degradation of AnNH by neurons involves the enzymatic hydrolysis of its amide bond with formation of arachidonic acid (AA) and ethanolamine. The enzyme catalyzing this hydrolysis, originally named ‘anandamide amidohydrolase’, was characterized from rat4, 5 and porcine6 brain membranes and was found in high levels also in the liver4. The enzyme was inhibited by typical serine and cysteine protease inhibitors, and displayed a peculiar pH dependency profile with optimal activity at 8.5<pH<10.0. The interest towards AnNH amidohydrolase was increased further by the suggestion2, 8 that the same enzyme may also catalyze the hydrolysis of other bioactive acylethanolamides, such as the anti-inflammatory palmitoylethanolamide (PEA)7, and the primary fatty acid amide, cis-9-octadecenoamide (oleamide), a novel sleep-inducing factor in mammals9. The recent cloning and expression of rat liver ‘oleamide hydrolase’ cDNA10 confirmed that the same enzyme can hydrolyze both oleamide and, at a higher rate, AnNH8, leading to propose for this enzyme the name ‘fatty acid amide hydrolase’ (FAAH)8, 10. In this paper we report the preliminary characterization of FAAH-like enzymatic activities from a blood cell type, the rat basophilic leukemia (RBL-1) cell line, and from other peripheral mammalian and invertebrate cells, and describe the effect of five synthetic AA-derived inhibitors on these enzymes as well as on the previously described amidohydrolases from mouse neuroblastoma (N18TG2) cells8 and porcine brain6.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A and Mechoulam R, Isolation and structure of a brain costituent that binds to the cannabinoid receptor, Science 258: 1946 (1992).
Di Marzo V, De Petrocellis L, Bisogno T and Maurelli S, Pharmacology and physiology of the endogenous cannabimimetic mediator anandamide, J Drug Dev Clin Pract 7: 199 (1995).
Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC and Piomelli D, Formation and inactivation of endogenous cannabinoid anandamide in central neurons, Nature 372: 686 (1994).
Desarnaud F, Cadas H and Piomelli D, Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization, J Biol Chem 270: 6030 (1995).
Hillard CJ, Wilkison DM, Edgemond WS and Campbell WB, Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain, Biochim Biophys Acta 1257: 249 (1995).
Ueda N, Kurahashi Y, Yamamoto S and Tokunaga T, Partial purification and characterization of the porcine brain enzyme hydrolizing and synthesizing anandamide, J Biol Chem 270: 23823 (1995).
Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD and Leon A, Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide, Proc Natl Acad Sci USA 92: 3376 (1995).
Maurelli S, Bisogno T, De Petrocellis L, Di Luccia A, Marino G and Di Marzo V, Two novel class of neuroactive fatty acid amides are substates for mouse neuroblastoma anandamide amidohydrolase, FEBS Lett 377: 82 (1995).
Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL and Lerner RA, Chemical characterization of a family of brain-lipids that induce sleep, Science 268: 1506 (1995).
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA and Gilula NB, Molecular characterization of an enzyme that degrades neuromodulatory fatty acid amides, Nature 384: 83 (1996).
Bisogno T, Ventriglia M, Mosca M, Milone A, Cimino G and Di Marzo V, Occurrence and metabolism of anandamide and related acylethanolamides in ovaries of the sea urchin Paracentrotus lividus, Biochim Biophys Acta (1997) in press.
De Petrocellis L, Melck D, Ueda N, Maurelli S, Kurahashi Y, Yamamoto S, Marino G and Di Marzo V, Novel inhibitors of brain, neuronal and basophilic anandamide aminohydrolase, Biochem Biophys Res Comm 231: 82 (1997).
Balsinde J and Dennis EA, Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages, J Biol Chem 271: 6758 (1996).
Koutek B, Prestwich GD, Howlett AC, Chin SA, Salehani D, Akhavan N and Deutsch DG, Inhibitors of arachidonyl ethanolamide hydrolisis, J Biol Chem 269: 22937 (1994).
Alanko J, Kurahashi Y, Yoshimoto T, Yamamoto S and Baba K, Panaxynol, a polyacetylene compound isolated from oriental medicine, inhibits mammalian lipoxygenases, Biochem Pharmacol 48: 1979 (1994).
Patterson JE, Ollmann IR, Cravatt BF, Boger DL, Wong C-H and Lerner RA, Inhibition of oleamide hydrolase catalyzed hydrolysis of the endogenous sleep-inducing lipid cis-9-octadecenoamide, J Am Chem Soc 118: 5938 (1996).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1997 Springer Science+Business Media New York
About this chapter
Cite this chapter
De Petrocellis, L. et al. (1997). Brain and Peripheral Anandamide Amidohydrolase and Its Inhibition by Synthetic Arachidonate Analogues. In: Sinzinger, H., Samuelsson, B., Vane, J.R., Paoletti, R., Ramwell, P., Wong, P.YK. (eds) Recent Advances in Prostaglandin, Thromboxane, and Leukotriene Research. Advances in Experimental Medicine and Biology, vol 433. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-1810-9_55
Download citation
DOI: https://doi.org/10.1007/978-1-4899-1810-9_55
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4899-1812-3
Online ISBN: 978-1-4899-1810-9
eBook Packages: Springer Book Archive