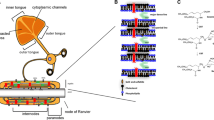
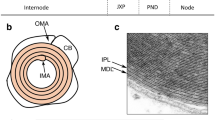
Abstract
For many years, myelin was the focal point for studies of membrane structure, largely because of its abundance and accessibility for chemical analyses, and its ordered laminar structure, which made it amenable to physical measurements. It is widely recognized that myelin is a highly specialized membrane, both in structure and in function, and that it should perhaps be thought of as an organelle derived from the plasma membrane of oligodendrocytes or Schwann cells as a result of cellular differentiation processes that occur during development of the nervous system. To gain some insight into the complexities of this membranous structure, it is important to relate the growing body of structural information on myelin to the general principles of membrane structure that have emerged from the numerous studies of other membrane systems. Thus, I have attempted in this chapter to discuss in a critical way those studies from which our current concepts of myelin structure have evolved and to frame this discussion in the broader context of membrane structure that is emerging from other investigations. No claim, however, is made for comprehensiveness in the citation of studies on myelin or in the review of supporting material, since this review is not intended to be an encyclopedic documentation of all contributions to the field.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Blaurock, A. E., 1981, The spaces between membrane bilayers within PNS myelin as characterized by X-ray diffraction, Brain Res. 210: 383.
Boggs, J. M., and Moscarello, M. A., 1978, Effect of basic protein from human CNS myelin on lipid bilayer structure, J. /14enzbr. Biol. 39: 75–96.
Boggs, J. M., Moscarello, M. A., and Papahadjopoulos, D., 1977, Phase separation of acidic and neutral phospholipids induced by human myelin basic protein, Biochemistry 16: 5420–5426.
Boggs, J. M., Wood, D. D., and Moscarello, M. A., 1981, Hydrophobic and electrostatic interactions of myelin basic protein with lipid: Participation of N-terminal and C-terminal protons, Biochemistry 20: 1065–1073.
Boggs, J. M., Clement, I. R., Moscarello, M. A., Eylar, E. H., and Haskin, G., 1981b, Antibody precipitation of lipid vesicles containing myelin proteins: Dependence on lipid composition, J. Immunology 126: 1207–1211.
Boggr., J. M., Moscarello, M. A., and Papahadjopoulos, D., 1982, Structural organization of myelin —Role of lipid-protein interactions determined in model systems, in: Lipid-Protein Interactions ( P. Jost and O. H. Griffith, eds.), pp. 1–51, Wiley, New York.
Brady, G. W., Birnbaum, P. S., Moscarello, M. A., and Papahadjopoulos, D., 1979, The model membrane system: Egg lecithin and myelin protein (N-2), effect of solvent density variation on the X-ray scattering, Biophys. J. 26: 49–60.
Braun, P. E., 1977, Molecular architecture of myelin, in: Myelin (Morell, ed.), Plenum Press, New York. Braun, P. E., and Radin, N. S., 1969, Interaction of lipids with a membrane structural protein from myelin, Biochemistry 8: 1310.
Braun, P. E., Frail, D. E., and I.aloe, N., 1982, Myelin-associated glycoprotein is the antigen for a monoclonal IgM in polyneuropathy, J. Neurochem. 39: 1261–1265.
Brinkley, B. R., 1981, Organization of the cytoplasm, Cold Spring Harbor Symp. Quant. Biol. 16: 1029–1040.
Capone, J., I.eblanc, P., Gerber, G. E., and Ghosh, H. P., 1983, Localization of membrane proteins by the use of a photoreactive fatty acid incorporated in vivo into vesicular stomatitis virus, J. Biol. Chem. 258: 1395–1398.
Chapman, B. E., and Moore, W. j., 1976, Conformation of myelin basic protein in aqueous solution from NMR spectroscopy, Biochem. Biophys. Res. Commun. 73: 758–766.
Chapman, D., and Wallach, D. F. H., 1968, Recent physical studies of phospholipids and natural membranes, in: Biological Membranes ( D. Chapman, ed.), pp. 125–202, Academic Press, New York.
Cherry, R. J., Mueller, U., Holenstein, C., and Heyn, M. P., 1980, Lateral segregation of proteins induced by cholesterol in bacteriorhodopsiu-phospholipid vesicles, BiocIiirn. Biophys. Acta 596: 145–151.
Cullen, M. J., DeVries, G. H., and Webster, H. de F., 1981, Freeze-fracture characterization of isolated myelin and axolemma membrane fractions, Brain Res. 229: 311–322.
Dawson, R. M. C., 1969, Metabolism and function of polyphosphionositides in nervous tissue, Ann. N. Y. Acad. Sci. 165: 774–783.
Dea, P., Chan, S. I., and Dea, F. J., 1972, High-resolution proton magnetic -resonance spectra of a rabbit sciatic nerve, Science 175: 206.
Dreiling, C. E., Schilling, R. J., and Reitz, R. C., 1981, 2’,3’-Cyclic nucleotide 3’-phosphohydrolase in rat liver mitochondrial membranes, Biochim. Biophys. Acta 640: 114–120.
Fisher, K. A., 1976, Analysis of membrane halves: Cholesterol, Proc. Natl. Acad. Sci. U.S.A. 73: 173–177.
Golds, E. E., and Braun, P. E., 1976, Organization of membrane proteins in the intact myelin sheath:
Pyridoxal phosphate and salicyladehyde as probes of myelin structure, J. Biol. Chem. 251:4729 Golds, E. E., and Braun, P. E., 1978a, Cross-linking studies on the conformation and dimerization of myelin basic protein in solution, J. Biol. Chem. 253:8171–8177.
Golds, E. E., and Braun, P. E., 1978b, Protein associations and basic protein conformation in the myelin membrane, J. Biol. Chem. 253:8162–8170
Gozes, I., and Richter-Landsberg, C., 1978, Identification of tubulin associated with rat brain myelin, FEBS Lett. 95: 169–172.
Griffith, O. H., Dehlinger, P. J., and Van, S. P., 1974, Shape of the hydrophobic barrier of phospholipid bilayers: Evidence for water penetration in biological membranes, J. Membr. Biol. 15: 159.
Guarnieri, M., 1975, Reaction of anti-phosphatidyl inositol antisera with neural membranes, Lipids 10: 294.
Hollingshead, C. J., Caspar, D. L. D., Melchior, V., and Kirschner, D. A., 1981, Compaction and particle segregation in myelin membrane arrays, J. Cell Biol. 89: 631.
Jones, A. J. S., and Rumbsy, M. G., 1977, Localization of sites for ionic interaction with lipid in the C-terminal third of the bovine myelin basic protein, Biochem. J. 167: 583–591.
Keniry, M. A., and Smith, R., 1979, Circular dichroia analysis of the secondary structure of myelin basic protein and derived peptides bound to detergents and to lipid vesicles, Biochim. Biophys. Acta. 578: 381–391.
Kirschner, D. A., and Ganser, A. L., 1982, Myelin labeled with mercuric chloride: Asymmetric localization of phosphatidylethanolamine plasmalogen, J. Mol. Biol. 157: 635–658.
Kirschner, D. A., Hollingshead, C. J., Thaxton, C., Caspar, D. L. D., and Goodenough, D. A., 1979, Structural states of myelin observed by X-ray diffraction and freeze-fracture electron microscopy, J. Cell Biol. 82: 140–149.
Ladbrooke, B. D., Jenkinson, T. J., Kama t, V. B., and Chapman, D., 1968, Physical studies of myelin. I. Thermal analysis, Biochim. Biophys. Acta 164: 101.
Lavialle, F., Foresta, B., Vacher, M., Nicot, C., and Alf sen, A., 1979, The molecular size and shape of the
Folch-Pi apoprotein in aqueous and organic solvents, Eur. J. Biochem. 95: 561–567.
Leblanc, P., Capone, J., and Gerber, G. E., 1982, Synthesis and biosynthetic utilization of radioactive photoreactive fatty acids, J. Biol. Chem. 257:14, 586–14, 589.
Liebes, L. F., Zand, R., and Phillips, W. D., 1975, Solution behavior, circular dichroism and 220 MHz
PMR studies of the bovine myelin basic protein, Biochim. Biophys. Acta 405:27–39.
Lin, L.-F., H., and Lees, M., 1982, Interaction of dicyclohexylcarbodiimide with myelin proteolipid, Proc. Natl. Acad. Sci. U.S.A. 79: 941.
Linington, C., and Rumsby, M. G., 1980, Accessibility of galactosyl ceramides to probe reagents in CNS myelin, J. Neurochem. 35: 983–992.
London, Y., Demel, R., Geurts Van Kessel, W. S. M., Vossenberg, F. G. A., and Van Deenen, L. L. M., 1973, The protection of a myelin basic protein against the action of proteolytic enzymes after interaction of the protein with lipids at the air-water interface, Biochim. Biophys. Acta 311: 520.
Mabrey, S., Mateo, P. L., and Sturtevant, J. M., 1978, High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl-and dipalmitoylphosphatidylcholines, Biochemistry 17: 2464–2468.
Macklin, W. B., Braun, P. E., and Lees, M., 1982, Electroblot analysis of the myelin proteolipid protein, J. Neurosci. Res. 7: 1–10.
Martenson, R. E., 1980, Myelin basic protein: What does it do?, in: Biochemistry of the Brain ( S. Kumar, ed.), pp. 47–79, Wiley, N. Y.
Matthieu, J. M., and Waehneldt, T. V., 1978, Protein and enzyme distribution in microsomal and myelin fractions from rat and Jimpy mouse brain, Brain Res. 150: 307–318.
McIntyre, R. J., Quarles, R. H., Webster, H. de F., and Brady, R. O., 1978, Isolation and characterization of myelin-related membranes, J. Neurochem. 30: 991–1002.
Moscarello, M. A., Gagnon, J., Wood, D. D., Anthony, J., and Epand, R. M., 1973, Conformational flexibility of a myelin protein, Biochemistry 12: 3402–3406.
Müller, H. W., Clapshaw, P. A., and Seifert, W., 1981, Intracellular localization of 2’,3’-cyclic nucleotide 3’-phosphodiesterase in a neuronal cell line as examined by immunofluorescence and cell fractionation, J. Neurochem. 37: 947–955.
Nagara, H., and Suzuki, K., 1982, Radial component of the central myelin in neurologic mutant mice, Lab. Invest. 47: 51–59.
O’Brien, J. S., 1965, Stability of the myelin membrane: Lipid molecules may impart stability to the myelin membrane through intermolecular cohesion, Science 147: 1099.
Omlin, F. X., Webster, H. de F., Palkoviti, G. G., and Cohen, S. R., 1982, Immunocytoehemieal localization of basic protein in major dense line regions of central and peripheral myelin, J. Cell Biol. 95: 242–248.
Palmer, F. B., and Dawson, R. M. C., 1969, Complex-formation between triphosphoinositide and experimental allergic encepholitogenic protein, Biochem. J. 111: 637.
Penman, S., Fulton, A., Capco, D., Ben Zeev, A., Wittelsberger, S., and Tse, C. F., 1982, Cytoplasmic and nuclear architecture in cells and tissue: Form, function and mode of assembly, Cold Spring Harbor Symp. Quant. Biol. 16: 1013–1028.
Pereyra, P. M., 1983, Ph.D. dissertation, McGill University. Studies on the mechanism of assembly of myelin in the CNS.
Peters, A., Palay, S. L., and Webster, H. de F., 1976, The Fine Structure of the Nervous System, W. B. Saunders, Philadelphia.
Peterson, R. G., and Sea, C. P., 1975, Ultrastructure and biochemistry of myelin after isoniazid-induced nerve degeneration in rats, Exptl. Neurol. 48: 252–260.
Peterson, R. G., and Gruener, R. W., 1978, Morphological localization of PNS myelin proteins, Brain Res. 152: 17–29.
Poduslo, J. F., and Braun, P. E., 1975, Topographical arrangement of membrane proteins in the intact myelin sheath, J. Biol. Chem. 250: 1099.
Poduslo, J. F., Quarles, R. II., and Brady, R. O., 1976, External labeling of galactose in surface membrane glycoproteins of the intact myelin sheath, J. Biol. Chem. 251: 153–158.
Quarles, R. H., 1980, Glycoproteins from central and peripheral myelin, in: Myelin: Chemistry and Biology ( G. A. Hashim, ed.), pp. 55–77, Alan Liss, New York.
Raine, C. S., Johnson, A. B., Marcus, D. M., Suzuki, A., and Bornstein, M., 1981, Demyelination in vitro:
Absorption studies demonstrate that galactocerebroside is a major target, J. Neural. Sc i. 52:117–131. Reiber, H., 1978, Cholesterol-lipid interactions in membranes: The saturation concentration of cholesterol in bilayers of various lipids, Biochim. Biophys. Acta 512: 72–83.
Reig, J. A. Ramos, J. M., Cozar, M., Aguilar, J. S., Criado, M., and Monreal, J., 1982, Purification and chemical characterization of a W2 protein from brain myelin, J. Neurochem. 39:507–511. Rothman, J. E., and Lenard, J., 1977, Membrane asymmetry, Science 195: 743–753.
Rumsby, M. G., and Crang, A. J., 1977, The myelin sheath—A structural examination, Cell Surf. Rev. 4: 247–362.
Sabatini, D. D., Kreibich, G., Morimoto,’1’., and Adesnik, M., 1982, Mechanisms for the incorporation of proteins in membranes and organelles, J. Cell Biol. 92: 1–22.
Sato, S., Quarles, R. H., and Brady, R. 0., 1982, Susceptibility of the myelin-associated glycoprotein and basic protein to a neutral protease in highly purified myelin from human and rat brain, J. Neurochem. 39: 97–105.
Schnapp, B., and Mugnaini, E., 1978, Membrane architecture of myelinated fibers as seen by freeze-fracture in: Physiology and Pathobiology of Axons (S. G. Waxman, ed.), pp. 83–123, Raven Press, New York.
Schreier, S., Polnaszek, C. F., and Smith, I. C. P., 1978, Spin labels in membranes: Problems in practice, Biochim. Biophys. Acta 515: 375–136.
Shapira, R., Môrley, W. C., Thiele, S. B., Wilhelmi, M. R., Wallace, A., and Kibler, R. F., 1978, Localization of 2’,3’-cyclic nucleotide 3’-phosphohydrolase of rabbit brain by sedimentation in a continuous sucrose gradient, J. Neurochem. 30: 735–744.
Singer, S. J., 1974, The molecular organization of membranes, Annu. Rev. Biochem. 43: 805.
Smith, R., 1977a, The secondary structure of myelin basic protein extracted by deoxycholate, Biochim. Biophys. Acta 491: 581–590.
Smith, R., 1977b, Non-covalent cross-linking of lipid bilayers by myelin basic protein: A possible role in myelin formation, Biochim. Biophys. Acta 470: 170–184.
Smith, R., 1980, Sedimentation analysis of the self-association of bovine myelin basic protein, Biochemistry 19: 1826–1831.
Smith, R., 1982, Self-association of myelin basic protein: Enhancement by detergents and lipids, Biochemistry 21: 2697–2701.
Smith, R., and McDonald, B. J., 1979, Association of myelin basic protein with detergent micelles, Biochim. Biophys. Acta 554: 133–147.
Smith, R., Cook, J., and Dickens, P. A., 1983, Structure of the proteolipid protein extracted from bovine CNS myelin with non-denaturing detergents. J. Neurochem. 42: 306–313.
Spacek, J., and Lieherman, A. R., 1980, The presence and possible significance of agranular reticulum in paranodal oligodendrocyte cytoplasm and in periglomerular astrocyte processes, Brain Res. 196: 498–501.
Steck, A. J., Siegrist, H. P., Zahler, P., and Hershkowitz, N. N., 1976, Lipid-protein interactions with native and modified basic protein, Biochim. Biophys. Acta. 455: 343–352.
Sternberger, N. H., Quarles, R. H., Itoyama, Y., and Webster, H. de F., 1979, Myelin-associated glycoproteins demonstrated immunocytochemically in myelin and myelin-forming cells of developing rat, Proc. Nall. Acad. Sei. U.S.A. 76: 1510–1514.
Tabira, T., and Webster, H. de F., 1979, E-PTA stains oligodendroglial surface membranes and microtubules in optic nerves during myelination, J. Neurol. Sei. 42: 215–227.
Tabira, T., Cullen, M. J., Reiff, P. J., and Webster, H. de F., 1978, An experimental analysis of interlammellar tight junctions in amphibian and mammalian CNS myelin, J. Neurocytol. 1: 489–503.
Trapp, B. D., and Quarks, R. H., 1982, Presence of the myelin-associated glycoprotein correlates with alterations in the periodicity of peripheral myelin, J. Cell Biol. 92: 877–882.
Trapp, B. D., McIntyre, L. J., Quarles, R. H., Sternberger, N. H., and Webster, H. de F., 1979, 1mmunoeytocheruica1 localization of rat PNS myelin proteins: P2 protein is not a component of all PNS myelin sheaths, Proc. Natl. Acad. Sci. U.S.A. 76: 3552–3556.
Trapp, B. D., Itoyama, Y., Macintosh, “F. D., and Quarles, R. H., 1983, P2 protein in oligodendrocvtes and myelin of the rabbit CNS, J. Neurochem. 40: 47–54.
Vandenheuvel, F. A., 1965, Study of biological structure at the molecular level with stereomodel projections. II. The structure of myelin in relation to other membrane systems, J. Ant. Oil Chem. Soc. 42: 481.
Wallach, D. F. H., and Winzler, R. J., 1974, Evolving Strategies and Tactics in Membrane Research, pp. 1–370, Springer-Verlag, New York.
Webster, H. de F., Palkovitz, C. G., Stoner, G. L., Favilla, J. T., Frail, D. E., and Braun, P. E., 1983, Electron microscopic immunocytochemical localization of myelin-associated glycoprotein in compact developing and adult CNS myelin, J. Neurochem. 41: 1469–1479.
Wiley, C. A., and Ellisman, M. H., 1980, Rows of dimeric particles within the axolemma and juxtaposed particles within glia, incorporated into a new model for the paranodal glial-axonal junction at the node of Ranvier, J. Cell Biol. 84: 261–280.
Wilson, L., and Margolis, R. L., 1981, Microtubule treadmills and their possible cellular functions, Cold Spring Harbor Syrup. Quant. Biol. 16: 199–205.
Wood, D. D., Epand, R. M., and Moscarello, M. A., 1977, Localization of the basic protein and lipophilin in the myelin membrane with a non-penetrating reagent, Biochim. Biophys. Acta 467: 120–129.
Wood, D. D., Boggs, J. M., and Moscarello, M. A., 1980, The transmembrane orientation of lipophilin in phosphatidylcholine vesicles, Neurochem. Res. 5: 745–756.
Wood, J. G., and McLaughlin, B. J., 1975, The visualizaiton of concanavalin-A binding sites in the interperiod line of rat sciatic nerve myelin, J. Neurochem. 24: 233.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1984 Springer Science+Business Media New York
About this chapter
Cite this chapter
Braun, P.E. (1984). Molecular Organization of Myelin. In: Morell, P. (eds) Myelin. Springer, Boston, MA. https://doi.org/10.1007/978-1-4757-1830-0_3
Download citation
DOI: https://doi.org/10.1007/978-1-4757-1830-0_3
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4757-1832-4
Online ISBN: 978-1-4757-1830-0
eBook Packages: Springer Book Archive