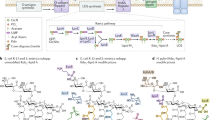
Abstract
Lipopolysaccharide (LPS) is a major constituent on the surface of the gram-negative bacteria including the bacteria of genus Yersinia. This chapter presents a short summary of the biosynthesis, genetics, and structural analysis of Yersinia LPS and then describes how the receptor structures of bacteriophages ϕR1-37 and ϕA1122 specific for Y. enterocolitica serotype O:3 and Y. pestis, respectively, were characterized.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Al-Hendy A (1992) Lipopolysaccharide of Yersinia enterocolitica: molecular genetics and role in virulence. PhD thesis. University of Turku, Turku, Finland
Al-Hendy A, Toivanen P, Skurnik M (1991) The effect of growth temperature on the biosynthesis of Yersinia enterocolitica O:3 lipopolysaccharide: temperature regulates the transcription of the rfb but not of the rfa region. Microb Pathog 10:81–86
Al-Hendy A, Toivanen P, Skurnik M (1992) Lipopolysaccharide O side chain of Yersinia enterocolitica O:3 is an essential virulence factor in an orally infected murine model. Infect Immun 60:870–875
Beczala A (2010) Immunochemical studies on lipopolysaccharide from Yersinia pseudotuberculosis O:9. University of Silesia, Department of Microbiology Katowice, Poland
Bengoechea JA, Skurnik M (2000) Temperature-regulated efflux pump/potassium antiporter system mediates resistance to cationic antimicrobial peptides in Yersinia. Mol Microbiol 37:67–80
Bengoechea JA, Pinta E, Salminen T et al (2002a) Functional characterization of Gne (UDP-N-acetylglucosamine-4-epimerase), Wzz (chain length determinant), and Wzy (O-antigen polymerase) of Yersinia enterocolitica serotype O:8. J Bacteriol 184:4277–4287
Bengoechea JA, Zhang L, Toivanen P et al (2002b) Regulatory network of lipopolysaccharide O-antigen biosynthesis in Yersinia enterocolitica includes cell envelope-dependent signals. Mol Microbiol 44:1045–1062
Bengoechea JA, Najdenski H, Skurnik M (2004) Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol Microbiol 52:451–469
Bogdanovich T, Carniel E, Fukushima H et al (2003) Use of O-antigen gene cluster-specific PCRs for the identification and O-genotyping of Yersinia pseudotuberculosis and Yersinia pestis. J Clin Microbiol 41:5103–5112
Bogdanovich T, Skurnik M, Lübeck PS et al (2004) Validated 5′ nuclease PCR assay for rapid identification of the genus Brucella. J Clin Microbiol 42:2261–2263
Carniel E, Autenrieth I, Cornelis G et al (2002) Y. enterocolitica and Y. pseudotuberculosis. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The Prokaryotes: an evolving electronic resource for the microbiological community. Springer-Verlag, New York
Cunneen MM, De Castro C, Kenyon J et al (2009) The O-specific polysaccharide structure and biosynthetic gene cluster of Yersinia pseudotuberculosis serotype O:11. Carbohydr Res 344:1533–1540
De Castro C, Skurnik M, Molinaro A et al (2009) Characterization of the specific O-polysaccharide structure and biosynthetic gene cluster of Yersinia pseudotuberculosis serotype O:15. Innate Immun 15:351–359
De Castro C, Kenyon JJ, Cunneen M et al (2010) Genetic characterisation and structural analysis of the O-specific polysaccharide of Yersinia pseudotuberculosis serotype O:1c. Innate Immun. doi:doi:10.1177/1753425910364425
De Castro C, Kenyon JJ, Cunneen MM et al (2011) Genetic characterisation and structural analysis of the O-specific polysaccharide of Yersinia pseudotuberculosis serotype O:1c. Innate Immun 17:183–190
Dentovskaya SV, Anisimov AP, Kondakova AN et al (2011) Functional characterization and biological significance of Yersinia pestis lipopolysaccharide biosynthesis genes. Biochemistry 76:808–822
Duda KA (2007a) Immunochemical studies on lipopolysaccharides from R mutants Yersinia enterocolitica O:3. University of Silesia, Department of Microbiology Katowice, Poland
Duda KT (2007b) Reactivity of polyclonal antisera against R mutants of Yersinia enterocolitica O:3. University of Silesia, Department of Microbiology Katowice, Poland
Duda KA, Duda KT, Beczala A et al (2009) ECA-immunogenicity of Proteus mirabilis strains. Arch Immunol Ther Exp 57:147–151
Filippov AA, Sergueev KV, He Y et al (2011) Bacteriophage-resistant mutants in Yersinia pestis: identification of phage receptors and attenuation for mice. PLoS ONE 6:e25486
Garcia E, Elliott JM, Ramanculov E et al (2003) The genome sequence of Yersinia pestis bacteriophage fA1122 reveals an intimate history with the coliphage T3 and T7 genomes. J Bacteriol 185:5248–5262
Gripenberg-Lerche C, Skurnik M, Zhang LJ et al (1994) Role of YadA in arthritogenicity of Yersinia enterocolitica serotype O:8: experimental studies with rats. Infect Immun 62:5568–5575
Gripenberg-Lerche C, Skurnik M, Toivanen P (1995a) Relation of YadA to arthritogenicity of Yersinia enterocolitica: experimental studies in the rat. In: Ravagnan G, Chiesa C (eds) Yersiniosis: present and future. Karger, Switzerland
Gripenberg-Lerche C, Skurnik M, Toivanen P (1995b) Role of YadA-mediated collagen binding in arthritogenicity of Yersinia enterocolitica serotype O:8: experimental studies with rats. Infect Immun 63:3222–3226
Gripenberg-Lerche C, Zhang L, Ahtonen P et al (2000) Construction of urease-negative mutants of Yersinia enterocolitica serotypes O:3 and O:8: role of urease in virulence and arthritogenicity. Infect Immun 68:942–947
Gronow S, Oertelt C, Ervelä E et al (2001) Characterization of the physiological substrate for lipopolysaccharide heptosyltransferases I and II. J Endotoxin Res 7:263–270
Jacobsen NR, Bogdanovich T, Skurnik M et al (2005) A real-time PCR assay for the specific identification of serotype O:9 of Yersinia enterocolitica. J Microbiol Methods 63:151–156
Jalava K, Hallanvuo S, Nakari UM et al (2004) Multiple outbreaks of Yersinia pseudotuberculosis infections in Finland. J Clin Microbiol 42:2789–2791
Kasperkiewicz K (2002) Wlasciwosci ECA immunogenne mutantów szorstkich Yersinia enterocolitica. Ph.D. Thesis (in Polish). University of Silesia, Department of Microbiology Katowice, Poland
Kenyon JJ, De Castro C, Cunneen MM et al (2011) The genetics and structure of the O-specific polysaccharide of Yersinia pseudotuberculosis serotype O:10 and its relationship with Escherichia coli O111 and Salmonella enterica O35. Glycobiology 21:1131–1139
Kiljunen S (2006) Molecular biology, genetics and applications of yersiniophages. University of Turku, Department of medical Biochemistry and Molecular biology Turku, Finland
Kiljunen S, Vilen H, Savilahti H et al (2003) Transposon mutagenesis of the phage fYeO3-12. In: Skurnik M, Granfors K, Bengoechea JA (eds) The Genus Yersinia: entering the functional genomic era. Kluwer Academic/Plenum Publishers, New York
Kiljunen S, Hakala K, Pinta E et al (2005a) Yersiniophage fR1-37 is a tailed bacteriophage having a 270 kb DNA genome with thymidine replaced by deoxyuridine. Microbiology 151:4093–4102
Kiljunen S, Vilen H, Pajunen M et al (2005b) Nonessential genes of phage fYeO3-12 include genes involved in adaptation to growth on Yersinia enterocolitica serotype O:3. J Bacteriol 187:1405–1414
Kiljunen S, Datta N, Dentovskaya SV et al (2011) Identification of the lipopolysaccharide core of Yersinia pestis and Yersinia pseudotuberculosis as the receptor for bacteriophage fA1122. J Bacteriol 193:4963–4972
Kondakova AN, Bystrova OV, Shaikhutdinova RZ et al (2008a) Reinvestigation of the O-antigens of Yersinia pseudotuberculosis: revision of the O2c and confirmation of the O3 antigen structures. Carbohydr Res 343:2486–2488
Kondakova AN, Ho N, Bystrova OV et al (2008b) Structural studies of the O-antigens of Yersinia pseudotuberculosis O:2a and mutants thereof with impaired 6-deoxy-d-manno-heptose biosynthesis pathway. Carbohydr Res 343:1383–1389
Kondakova AN, Bystrova OV, Shaikhutdinova RZ et al (2009a) Structure of the O-antigen of Yersinia pseudotuberculosis O:4a revised. Carbohydr Res 344:531–534
Kondakova AN, Bystrova OV, Shaikhutdinova RZ et al (2009b) Structure of the O-polysaccharide of Yersinia pseudotuberculosis O:2b. Carbohydr Res 344:405–407
Kondakova AN, Bystrova OV, Shaikhutdinova RZ et al (2009c) Structure of the O-antigen of Yersinia pseudotuberculosis O:4b. Carbohydr Res 344:152–154
Kondakova AN, Shaikhutdinova RZ, Ivanov SA et al (2009d) Revision of the O-polysaccharide structure of Yersinia pseudotuberculosis O:1b. Carbohydr Res 344:2421–2423
Matero P, Pasanen T, Laukkanen R et al (2009) Real-time multiplex PCR assay for detection of Yersinia pestis and Yersinia pseudotuberculosis. APMIS 117:34–44
Müller-Loennies S, Rund S, Ervelä E et al (1997) Structural analysis of the core-lipid A region of the lipopolysaccharide from a rough mutant of Yersinia enterocolitica O:9. 9th European carbohydrate symposium, Ütrecht, July 6–11, 1997
Müller-Loennies S, Rund S, Ervelä E et al (1999) The structure of the carbohydrate backbone of the core-lipid A region of the lipopolysaccharide from a clinical isolate of Yersinia enterocolitica O:9. Eur J Biochem 261:19–24
Muszynski A (2004) Characterisation of lipopolysaccharides from mutants of Yersinia enterocolitica O:3 cultivated at different temperatures. University of Silesia, Department of Microbiology, Katowice, Poland
Najdenski H, Golkocheva E, Vesselinova A et al (2003) Proper expression of the O-antigen of lipopolysaccharide is essential for the virulence of Yersinia enterocolitica O:8 in experimental oral infection of rabbits. FEMS Immunol Med Microbiol 38:97–106
Najdenski H, Golkocheva E, Kussovski V et al (2006) Experimental pig yersiniosis to assess attenuation of Yersinia enterocolitica O:8 mutant strains. FEMS Immunol Med Microbiol 47:425–435
Oertelt C (2001) Studies on the structure and biosynthesis of the lipopolysaccharide from Yersinia enterocolitica serotype O:8. Medical University of Lübeck, Lübeck, Germany
Oertelt C, Lindner B, Skurnik M et al (2001) Isolation and structural characterization of an R-form lipopolysaccharide from Yersinia enterocolitica serotype O:8. Eur J Biochem 268:554–564
Oyston PC, Prior JL, Kiljunen S et al (2003) Expression of heterologous O-antigen in Yersinia pestis KIM does not affect virulence by the intravenous route. J Med Microbiol 52:289–294
Pajunen M (2001) Molecular analysis of Yersinia enterocolitica serotype O:3 -specific bacteriophage fYeO3-12. University of Turku, Department of Medical Biochemistry Turku, Finland
Pajunen M, Kiljunen S, Skurnik M (2000) Bacteriophage fYeO3-12, specific for Yersinia enterocolitica serotype O:3, is related to coliphages T3 and T7. J Bacteriol 182:5114–5120
Pajunen MI, Kiljunen SJ, Söderholm MEL et al (2001) Complete genomic sequence of the lytic bacteriophage fYeO3-12 of Yersinia enterocolitica serotype O:3. J Bacteriol 183:1928–1937
Pajunen MI, Elizondo MR, Skurnik M et al (2002) Complete nucleotide sequence and likely recombinatorial origin of bacteriophage T3. J Mol Biol 319:1115–1132
Pajunen MI, Molineux IJ, Skurnik M (2003) Yersiniophages. In: Skurnik M, Granfors K, Bengoechea JA (eds) The Genus Yersinia: entering the functional genomic era. Kluwer Academic/Plenum Publishers, New York
Perez-Gutierrez C, Llompart CM, Skurnik M et al (2007) Expression of the Yersinia enterocolitica pYV-encoded type III secretion system is modulated by lipopolysaccharide O-antigen status. Infect Immun 75:1512–1516
Pinta E (2010) Biosynthesis of Yersinia enterocolitica serotype O:3 lipopolysaccharide outer core. University of Turku, Department of Medical Biochemistry and genetics Turku, Finland
Pinta E, Duda KA, Hanuszkiewicz A et al (2009) Identification and role of a 6-deoxy-4-keto-hexosamine in the lipopolysaccharide outer core of Yersinia enterocolitica serotype O:3. Chemistry 15:9747–9754
Pinta E, Duda KA, Hanuszkiewicz A et al (2010) Characterization of the six glycosyltransferases involved in the biosynthesis of Yersinia enterocolitica serotype O:3 lipopolysaccharide outer core. J Biol Chem 285:28333–28342
Pinta E, Li Z, Batzilla J et al (2012) Identification of three oligo-/polysaccharide-specific ligases in Yersinia enterocolitica. Mol Microbiol 83:125–136.
Rabsztyn K, Kasperkiewicz K, Duda KA et al (2011) Characterization of anti-ECA antibodies in rabbit antiserum against rough Yersinia enterocolitica O:3. Biochemistry 76:832–839
Radziejewska-Lebrecht J, Skurnik M, Shashkov AS et al (1998) Immunochemical studies on R mutants of Yersinia enterocolitica O:3. Acta Biochim Pol 45:1011–1019
Radziejewska-Lebrecht J, Kasperkiewicz K, Skurnik M et al (2003a) ECA-antibodies in antisera against R mutants of Yersinia enterocolitica O:3. Adv Exp Med Biol 529:215–218
Radziejewska-Lebrecht J, Kasperkiewicz K, Skurnik M et al (2003b) ECA-antibodies in antisera against R mutants of Yersinia enterocolitica O:3. In: Skurnik M, Granfors K, Bengoechea JA (eds) The Genus Yersinia: entering the functional genomic era. Kluwer Academic/Plenum Publishers, New York
Rosqvist R, Skurnik M, Wolf-Watz H (1988) Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature 334:522–525
Ruiz N, Kahne D, Silhavy TJ (2009) Transport of lipopolysaccharide across the cell envelope: the long road of discovery. Nat Rev Microbiol 7:677–683
Skurnik M (1999) Molecular genetics of Yersinia lipopolysaccharide. In: Goldberg J (ed) Genetics of bacterial polysaccharides. CRC Press, Boca Raton
Skurnik M (2003) Molecular genetics, biochemistry and biological role of Yersinia lipopolysaccharide. In: Skurnik M, Granfors K, Bengoechea JA (eds) The Genus Yersinia: entering the functional genomic era. Kluwer Academic/Plenum Publishers, New York
Skurnik M (2004) Lipopolysaccharides of Yersinia. In: Carniel E, Hinnebusch BJ (eds) Yersinia: molecular and cellular biology. Horizon Bioscience, Wymondham
Skurnik M (2007) My life with Yersinia. Adv Exp Med Biol 603:44–73
Skurnik M, Bengoechea JA (2003) The biosynthesis and biological role of lipopolysaccharide O-antigens of pathogenic Yersiniae. Carbohydr Res 338:2521–2529
Skurnik M, Bengoechea JA (2009) Genetics and regulation of bacterial lipopolysaccharide synthesis. In: Ullrich M (ed) Bacterial polysaccharides—current innovations and future trends. Caister Academic Press, Norfolk
Skurnik M, Poikonen K (1986) Experimental intestinal infection of rats by Yersinia enterocolitica O:3. A follow-up study with specific antibodies to the virulence plasmid specified antigens. Scand J Inf Dis 18:355–364
Skurnik M, Strauch E (2006) Phage therapy: facts and fiction. Int J Med Microbiol 296:5–14
Skurnik M, Toivanen P (1993) Yersinia enterocolitica lipopolysaccharide: genetics and virulence. Trends Microbiol 1:148–152
Skurnik M, Zhang L (1996) Molecular genetics and biochemistry of Yersinia lipopolysaccharide. APMIS 104:849–872
Skurnik M, Venho R, Toivanen P et al (1995) A novel locus of Yersinia enterocolitica serotype O:3 involved in lipopolysaccharide outer core biosynthesis. Mol Microbiol 17:575–594
Skurnik M, Venho R, Bengoechea JA et al (1999) The lipopolysaccharide outer core of Yersinia enterocolitica serotype O:3 is required for virulence and plays a role in outer membrane integrity. Mol Microbiol 31:1443–1462
Skurnik M, Peippo A, Ervelä E (2000) Characterization of the O-antigen gene clusters of Yersinia pseudotuberculosis and the cryptic O-antigen gene cluster of Yersinia pestis shows that the plague bacillus is most closely related to and has evolved from Y. pseudotuberculosis serotype O:1b. Mol Microbiol 37:316–330
Stolen CM, Marttila-Ichihara F, Koskinen K et al (2005) Absence of the endothelial oxidase AOC3 leads to abnormal leukocyte traffic in vivo. Immunity 22:105–115
Tamm A, Tarkkanen AM, Korhonen TK et al (1993) Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol Microbiol 10:995–1011
Uliczka F, Pisano F, Schaake J et al (2011) Unique cell adhesion and invasion properties of Yersinia enterocolitica O:3, the most frequent cause of human Yersiniosis. PLoS Pathog 7:e1002117
Zhang L, Skurnik M (1994) Isolation of an R- M + mutant of Yersinia enterocolitica serotype O:8 and its application in construction of rough mutants utilizing mini-Tn5 derivatives and lipopolysaccharide-specific phage. J Bacteriol 176:1756–1760
Zhang L, Radziejewska-Lebrecht J, Krajewska-Pietrasik D et al (1997) Molecular and chemical characterization of the lipopolysaccharide O-antigen and its role in the virulence of Yersinia enterocolitica serotype O:8. Mol Microbiol 23:63–76
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media New York
About this paper
Cite this paper
Skurnik, M. (2012). Yersinia Surface Structures and Bacteriophages. In: de Almeida, A., Leal, N. (eds) Advances in Yersinia Research. Advances in Experimental Medicine and Biology, vol 954. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-3561-7_37
Download citation
DOI: https://doi.org/10.1007/978-1-4614-3561-7_37
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-3560-0
Online ISBN: 978-1-4614-3561-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)