Abstract
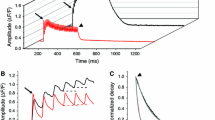
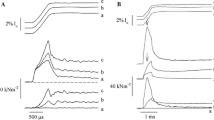
Muscle relaxation occurs when Ca2+, sequestrated by the sarcoplasmic reticulum (SR) Ca2+ pump, dissociates from troponin (Tn) to deactivate the thin filaments leading to cross-bridge detachment and force decay. It is well established that the rate of Ca2+ sequestration by the SR can control relaxation kinetics.1 The aim of the present investigation is to determine the relative contribution of Ca2+ dissociation from TnC and cross-bridge detachment to relaxation rate induced by rapid sequestration of Ca2+. Three possibilities can be envisioned. First, Ca2+ dissociation from TnC may be much faster than cross-bridge detachment. In this case, only cross-bridge detachment kinetics would affect relaxation rate. Second, Ca2+ dissociation from TnC may be similar to cross-bridge detachment. If this relationship were true, both cross-bridge and TnC kinetics would affect relaxation rate. Third, Ca2+ dissociation from TnC may be slower than cross-bridge detachment. If this possibility were true, then only the kinetics of Ca2+ dissociation from TnC would affect relaxation rate.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
J. M. Gillis, Relaxation of vertebrate skeletal muscle, Biochim. Biophys. Acta 811, 97–145 (1985).
Y. Luo, J. P. Davis, L B. Smillie, and J. A. Rail, Determinants of relaxation rate in rabbit skinned skeletal muscle fibres. J. Physiol. in press (2003).
C. Tesi, N. Piroddi, F. Colomo, and C. Poggesi, Relaxation kinetics following sudden Ca2+ reduction in single myofibrils from skeletal muscle, Biophys. J. 83, 2142–2151 (2002).
P. A. Wahr, J. D. Johnson, and J. A. Rail, Determinants of relaxation rate in skinned frog skeletal muscle fibers, Am. J. Physiol. 274, C1608–C1615 (1998).
S. R. Adams, J. P. Kao, and R. Y. Tsien, Biological useful chelators that take up Ca2+ upon illumination, J. Am. Chem. Soc. 111, 7957–7968 (1989).
M. J. Brune, L. Hunter, E. T. Corrie, and M. R. Webb, Direct, real time measurements of rapid inorganic phosphate release using a novel fluorescent probe and its application to actomyosin subfragment 1 ATPase, Biochem. 33, 8262–8271 (1994).
N. C. Millar and E. Homsher, The effects of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. J. Biol. Chem. 265, 20234–20240 (1990).
E. Pate, K. Franks-Skiba, and R. Cooke, Depletion of phosphate in active muscle fibers probes actomyosin states within the powerstroke. Biophys. J. 74, 369–380 (1998).
C. Tesi, F. Colomo, S. Nencini, N. Piroddi, and C. Poggesi, The effects of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophys. J. 78, 3081–3092 (2000).
M Chandra, E. F. da Silva, M. M. Sorenson, J. A. Ferro, J. R. Pearlstone, B. E. Nash, T. Borgford, C. M. Kay, and L. B. Smillie, The effects of N helix deletion and mutant F29W on the Ca2+ binding and functional properties of chicken skeletal muscle troponin C. J. Biol Chem. 269, 14988–14994 (1994).
J. R. Pearlstone, T. Borgford, M. Chandra, K. Oilawa, C. M. Kay, O. Herzberg, J. Moult, J., A. Herklotz, F. C. Reinach, and L. B. Smillie, Construction and characterization of a spectral probe mutant of troponin C: application to analyses of mutants with increased Ca2+ affinity. Biochem. 31, 6545–6553, 1992.
P. Mulligan, R. E. Palmer, S. Lipscomb, B. Hoskins, and C. C. Ashley, The effect of phosphate on the relaxation of frog skeletal muscle. Pflug. Arch. 437, 393–399 (1999).
J. M. Metzger, M. L. Greaser, and R. L. Moss, Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. J. Gen. Physiol. 93, 855–883 (1989).
S. B. Tikunova, J. A. Rall, and J. P. Davis, Effects of hydrophobic residue substitutions with glutamine on Ca2+ binding and exchange with the N-domain of troponin C. Biochem. 41, 6697–6705, 2002.
J. P. Davis, S. B. Tikunova, C. Alionte, and J. A. Rail, Conserved Hydrophobic β-Sheet and β-Sheet Interacting Residues Are Critical for Troponin C (TnC) Function in Skeletal Muscle. Biophys. J. in press (2003).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2003 Springer Science+Business Media New York
About this paper
Cite this paper
Luo, Y., Davis, J.P., Tikunova, S.B., Smillie, L.B., Rall, J.A. (2003). Myofibrillar Determinants of Rate of Relaxation in Skinned Skeletal Muscle Fibers. In: Sugi, H. (eds) Molecular and Cellular Aspects of Muscle Contraction. Advances in Experimental Medicine and Biology, vol 538. Springer, Boston, MA. https://doi.org/10.1007/978-1-4419-9029-7_51
Download citation
DOI: https://doi.org/10.1007/978-1-4419-9029-7_51
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4613-4764-4
Online ISBN: 978-1-4419-9029-7
eBook Packages: Springer Book Archive