Abstract
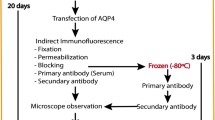
Cell-based assay (CBA) is an immunofluorescence assay that is extensively used for the confirmatory diagnosis of inflammatory demyelinating diseases of the central nervous system, like neuromyelitis optica spectrum disorder (NMOSD). Detecting the type of autoantibody present in the sera of the patients is the primary goal. CBA is the most sensitive and recommended detection method among all similar tools. Briefly, serum autoantibody is screened by transfecting specific cells seeded on cover glasses with full-length specific antigen fused with green fluorescent protein (GFP), followed by treating them with the patient serum used here as the source of primary antibody. The autoantibody-treated cells are further labeled with a rhodamine-conjugated secondary antibody. The co-localization of GFP and rhodamine is visualized by confocal microscopy, and the intensity of fluorescence is evaluated to determine the presence of autoantibody. A detailed protocol to screen antibodies against AQP4 and MOG in human sera using this method is described.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, Jarius S, Lana-Peixoto M, Levy M, Simon JH, Tenembaum S, Traboulsee AL, Waters P, Wellik KE, Weinshenker BG (2015) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85:177–189
Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, Palace J, Vincent A (2012) Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 79:1273–1277
Bernard-Valnet R, Liblau RS, Vukusic S, Marignier R (2015) Neuromyelitis optica: a positive appraisal of seronegative cases. Eur J Neurol 22:1511–1e83
Hamid SHM, Whittam D, Mutch K, Linaker S, Solomon T, Das K, Bhojak M, Jacob A (2017) What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol 264:2088–2094
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364:2106–2112
Mader S, Lutterotti A, Di Pauli F, Kuenz B, Schanda K, Aboul-Enein F, Khalil M, Storch MK, Jarius S, Kristoferitsch W, Berger T, Reindl M (2010) Patterns of antibody binding to aquaporin-4 isoforms in neuromyelitis optica. PLoS One 5:e10455
Ruiz-Gaviria R, Baracaldo I, Castañeda C, Ruiz-Patiño A, Acosta-Hernandez A, Rosselli D (2015) Specificity and sensitivity of aquaporin 4 antibody detection tests in patients with neuromyelitis optica: a meta-analysis. Mult Scler Relat Disord 4:345–349
Pisani F, Sparaneo A, Tortorella C, Ruggieri M, Trojano M, Mola MG, Nicchia GP, Frigeri A, Svelto M (2013) Aquaporin-4 autoantibodies in neuromyelitis optica: aqp4 isoform-dependent sensitivity and specifcity. PLoS One 8:e79185
Waters P, Reindl M, Saiz A, Schanda K, Tuller F, Kral V, Nytrova P, Sobek O, Nielsen HH, Barington T, Lillevang ST, Illes Z, Rentzsch K, Berthele A, Berki T, Granieri L, Bertolotto A, Giometto B, Zuliani L, Hamann D, van Pelt ED, Hintzen R, Höftberger R, Costa C, Comabella M, Montalban X, Tintoré M, Siva A, Altintas A, Deniz G, Woodhall M, Palace J, Paul F, Hartung HP, Aktas O, Jarius S, Wildemann B, Vedeler C, Ruiz A, Leite MI, Trillenberg P, Probst M, Saschenbrecker S, Vincent A, Marignier R (2016) Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry 87:1005–1015
Mader S, Gredler V, Schanda K, Rostasy K, Dujmovic I, Pfaller K, Lutterotti A, Jarius S, Di Pauli F, Kuenz B, Ehling R, Hegen H, Deisenhammer F, Aboul-Enein F, Storch MK, Koson P, Drulovic J, Kristoferitsch W, Berger T, Reindl M (2011) Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 8:184
Chan KH, Kwan JS, Ho PW, Ho JW, Chu AC, Ramsden DB (2010) Aquaporin-4 autoantibodies in neuromyelitis optica spectrum disorders: comparison between tissue-based and cell-based indirect immunofluorescence assays. J Neuroinflamm 7:50
Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, Nakamura M, Watanabe S, Ishii N, Itoyama Y (2006) Establishment of a new sensitive assay for anti-human aquaporin-4 antibody in neuromyelitis optica. Tohoku J Exp Med 210:307–313
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Chatterjee, P., Saha, S., Mukhopadhyay, D. (2024). Cell-Based Assay to Detect the Autoantibody Serostatus in Patients with Neuromyelitis Optica Spectrum Disorder (NMOSD). In: Ray, S.K. (eds) Neuroprotection. Methods in Molecular Biology, vol 2761. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3662-6_10
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3662-6_10
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3661-9
Online ISBN: 978-1-0716-3662-6
eBook Packages: Springer Protocols