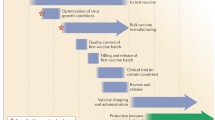
Abstract
Cold-adapted Ann Arbor based live attenuated influenza vaccine (LAIV) has been available in the USA since 2003. The vaccine is efficacious against influenza infection. Features of LAIV include: easy administration suitable for mass immunization, cross-reactivity to drifted strains for broader coverage, and establishment of herd immunity for control of influenza spread. Annual seasonal LAIV now contains four strains against influenza A H1N1, H3N2, influenza B-Victoria, and B-Yamagata lineages that are co-circulating in humans. LAIV played a significant role in protecting the public from the 2009 H1N1 pandemic and has been evaluated for pandemic preparedness. Pandemic vaccines including influenza H2, H5, H6, H7, and H9 subtypes have been produced and evaluated in preclinical and small-scale phase I clinical studies. This review summarizes the current status and perspectives of seasonal and pandemic LAIV.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- LAIV :
-
Live attenuated influenza vaccine
- T/LAIV :
-
Trivalent live attenuated influenza vaccine
- Q/LAIV :
-
Quadrivalent live attenuated influenza vaccine
- pLAIV :
-
Pandemic live attenuated influenza vaccine
- TIV:
-
Trivalent inactivated influenza vaccine
- IIV:
-
Inactivated influenza vaccine
- MDV-A:
-
Master donor virus for influenza A vaccines
- MDV-B:
-
Master donor virus for influenza B vaccines
- ca :
-
Cold adapted
- ts :
-
Temperature sensitive
- att :
-
Attenuated
- wt :
-
Wild-type
- RG:
-
Reverse genetics
- PCKC:
-
Primary chicken kidney cells
- NT:
-
Nasal turbinates
- HAI:
-
Hemagglutination inhibition
- NW:
-
Nasal wash
References
Ambrose CS, Luke C, Coelingh K (2008) Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. Influenza Other Respir Viruses 2:193–202
Ambrose CS, Wu X, Knuf M, Wutzler P (2012) The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: a meta-analysis of 8 randomized controlled studies. Vaccine 30:886–892
Ambrose CS, Yi T, Falloon J (2011) An integrated, multistudy analysis of the safety of Ann Arbor strain live attenuated influenza vaccine in children aged 2–17 years. Influenza Other Respir Viruses 5:389–397
Bandell A, Woo J, Coelingh K (2011) Protective efficacy of live-attenuated influenza vaccine (multivalent, Ann Arbor strain): a literature review addressing interference. Expert Rev Vaccines 10:1131–1141
Baxter R, Toback SL, Sifakis F, Hansen J, Bartlett J, Aukes L, Lewis N, Wu X, Ambrose CS (2012a) A postmarketing evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in adults 18–49 years of age. Vaccine 30:3053–3060
Baxter R, Toback SL, Sifakis F, Hansen J, Bartlett J, Aukes L, Lewis N, Wu X, Ambrose CS (2012b) A postmarketing evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in children 5 through 17 years of age. Vaccine 30:2989–2998
Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM (2007) Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 356:685–696
Belshe RB, Gruber WC, Mendelman PM, Cho I, Reisinger K, Block SL, Wittes J, Iacuzio D, Piedra P, Treanor J, King J, Kotloff K, Bernstein DI, Hayden FG, Zangwill K, Yan L, Wolff M (2000) Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr 136:168–175
Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, Bernstein DI, Hayden FG, Kotloff K, Zangwill K, Iacuzio D, Wolff M (1998) The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med 338:1405–1412
Belshe RB, Nichol KL, Black SB, Shinefield H, Cordova J, Walker R, Hessel C, Cho I, Mendelman PM (2004) Safety, efficacy, and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5–49 years. Clin Infect Dis. 39:920–927
Beyer WE, Palache AM, de Jong JC, Osterhaus AD (2002) Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine 20:1340–1353
Cha TA, Kao K, Zhao J, Fast PE, Mendelman PM, Arvin A (2000) Genotypic stability of cold-adapted influenza virus vaccine in an efficacy clinical trial. J Clin Microbiol 38:839–845
Chan W, Zhou H, Kemble G, Jin H (2008) The cold adapted and temperature sensitive influenza A/Ann Arbor/6/60 virus, the master donor virus for live attenuated influenza vaccines, has multiple defects in replication at the restrictive temperature. Virology 380:304–311
Chen GL, Lamirande EW, Cheng X, Torres-Velez F, Orandle M, Jin H, Kemble G, Subbarao K (2014) Evaluation of three live attenuated h2 pandemic influenza vaccine candidates in mice and ferrets. J Virol 88:2867–2876
Chen GL, Lamirande EW, Jin H, Kemble G, Subbarao K (2009a) Safety, immunogencity, and efficacy of a cold-adapted A/Ann Arbor/6/60 (H2N2) vaccine in mice and ferrets. Virology 398:109–114
Chen Z, Santos C, Aspelund A, Gillim-Ross L, Jin H, Kemble G, Subbarao K (2009b) Evaluation of live attenuated influenza a virus H6 vaccines in mice and ferrets. J Virol 83:65–72
Chen GL, Lau YF, Lamirande EW, McCall AW, Subbarao K (2011a) Seasonal influenza infection and live vaccine prime for a response to the 2009 pandemic H1N1 vaccine. Proc Natl Acad Sci USA 108:1140–1145
Chen GL, Min JY, Lamirande EW, Santos C, Jin H, Kemble G, Subbarao K (2011b) Comparison of a live attenuated 2009 H1N1 vaccine with seasonal influenza vaccines against 2009 pandemic H1N1 virus infection in mice and ferrets. J Infect Dis 203:930–936
Chen H, Matsuoka Y, Swayne D, Chen Q, Cox NJ, Murphy BR, Subbarao K (2003) Generation and characterization of a cold-adapted influenza A H9N2 reassortant as a live pandemic influenza virus vaccine candidate. Vaccine 21:4430–4436
Chen Z, Aspelund A, Kemble G, Jin H (2008) Molecular studies of temperature-sensitive replication of the cold-adapted B/Ann Arbor/1/66, the master donor virus for live attenuated influenza FluMist vaccines. Virology 380:354–362
Chen Z, Wang W, Zhou H, Suguitan AL Jr, Shambaugh C, Kim L, Zhao J, Kemble G, Jin H (2010a) Generation of live attenuated novel influenza virus A/California/7/09 (H1N1) vaccines with high yield in embryonated chicken eggs. J Virol 84:44–51
Chen Z, Zhou H, Jin H (2010b) The impact of key amino acid substitutions in the hemagglutinin of influenza A (H3N2) viruses on vaccine production and antibody response. Vaccine 28:4079–4085
Cheng X, Zengel JR, Suguitan AL Jr, Xu Q, Wang W, Lin J, Jin H (2013) Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J Infect Dis 208:594–602
Clements ML, Betts RF, Tierney EL, Murphy BR (1986) Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol 24:157–160
Coelingh K, Talaat KR, Luke CJ, Jin H, Karron RA (2014) Development of live attenuated influenza vaccines against pandemic influenza strains. Expert Rev Vaccines 7:855–871
Cox RJ, Brokstad KA, Ogra P (2004) Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 59:1–15
De Villiers PJ, Steele AD, Hiemstra LA, Rappaport R, Dunning AJ, Gruber WC, Forrest BD, Network LEST (2009) Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older. Vaccine 28:228–234
Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF (1994) A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis 169:68–76
Forrest BD, Pride MW, Dunning AJ, Capeding MR, Chotpitayasunondh T, Tam JS, Rappaport R, Eldridge JH, Gruber WC (2008) Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol 15:1042–1053
Gorse GJ, Campbell MJ, Otto EE, Powers DC, Chambers GW, Newman FK (1995) Increased anti-influenza A virus cytotoxic T cell activity following vaccination of the chronically ill elderly with live attenuated or inactivated influenza virus vaccine. J Infect Dis 172:1–10
Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ (2006) Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev 28:3–26
He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, Dekker CL, Greenberg HB, Arvin AM (2006) Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol 80:11756–11766
Herberg JA, Kaforou M, Gormley S, Sumner ER, Patel S, Jones KD, Paulus S, Fink C, Martinon-Torres F, Montana G, Wright VJ, Levin M (2013) Transcriptomic profiling in childhood H1N1/09 influenza reveals reduced expression of protein synthesis genes. J Infect Dis 208:1664–1668
Hoffmann E, Mahmood K, Chen Z, Yang CF, Spaete J, Greenberg HB, Herlocher ML, Jin H, Kemble G (2005) Multiple gene segments control the temperature sensitivity and attenuation phenotypes of ca B/Ann Arbor/1/66. J Virol 79:11014–11021
Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, Abate G, Sakala IG, Edwards KM, Creech CB, Gerber MA, Bernstein DI, Newman F, Graham I, Anderson EL, Belshe RB (2011) Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis 204:845–853
Jin H, Chen Z (2014) Production of live attenuated influenza vaccines against seasonal and pandemic influenza viruses. Curr Opin Virol 6:34–39
Jin H, Lu B, Zhou H, Kemble G (2004) Genetic studies of FluMist influenza vaccines derived from cold adapted A/Ann Arbor/6/60, options for the control of inluenza V. Elsevier, Okinawa. In: Kawaoka Y (ed), Int Congr Ser 1263, pp 153–156
Jin H, Lu B, Zhou H, Ma C, Zhao J, Yang CF, Kemble G, Greenberg H (2003) Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology 306:18–24
Joseph T, McAuliffe J, Lu B, Vogel L, Swayne D, Jin H, Kemble G, Subbarao K (2008) A live attenuated cold-adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology 378:123–132
Karron RA, Callahan K, Luke C, Thumar B, McAuliffe J, Schappell E, Joseph T, Coelingh K, Jin H, Kemble G, Murphy BR, Subbarao K (2009a) A live attenuated H9N2 influenza vaccine is well tolerated and immunogenic in healthy adults. J Infect Dis 199:711–716
Karron RA, Talaat K, Luke C, Callahan K, Thumar B, Dilorenzo S, McAuliffe J, Schappell E, Suguitan A, Mills K, Chen G, Lamirande E, Coelingh K, Jin H, Murphy BR, Kemble G, Subbarao K (2009b) Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine 27:4953–4960
Lanthier PA, Huston GE, Moquin A, Eaton SM, Szaba FM, Kummer LW, Tighe MP, Kohlmeier JE, Blair PJ, Broderick M, Smiley ST, Haynes L (2011) Live attenuated influenza vaccine (LAIV) impacts innate and adaptive immune responses. Vaccine 29:7849–7856
Lau YF, Santos C, Torres-Velez FJ, Subbarao K (2011) The magnitude of local immunity in the lungs of mice induced by live attenuated influenza vaccines is determined by local viral replication and induction of cytokines. J Virol 85:76–85
Lau YF, Wright AR, Subbarao K (2012) The contribution of systemic and pulmonary immune effectors to vaccine-induced protection from H5N1 influenza virus infection. J Virol 86:5089–5098
Longini IM Jr, Koopman JS, Monto AS, Fox JP (1982) Estimating household and community transmission parameters for influenza. Am J Epidemiol 115:736–751
Maassab HF (1967) Adaptation and growth characteristics of influenza virus at 25 degrees C. Nature 213:612–614
Min JY, Vogel L, Matsuoka Y, Lu B, Swayne D, Jin H, Kemble G, Subbarao K (2010) A live attenuated H7N7 candidate vaccine virus induces neutralizing antibody that confers protection from challenge in mice, ferrets, and monkeys. J Virol 84:11950–11960
Monto AS, Ohmit SE, Petrie JG, Johnson E, Truscon R, Teich E, Rotthoff J, Boulton M, Victor JC (2009) Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 361:1260–1267
Murphy BR, Coelingh K (2002) Principles underlying the development and use of live attenuated cold-adapted influenza A and B virus vaccines. Viral Immunol 15:295–323
Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, Glezen WP, Wittes J (1999a) Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA 282:137–144
Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, Glezen WP, Wittes J (1999b) Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial [in process citation]. JAMA 282:137–144
Renegar KB, Small PA Jr, Boykins LG, Wright PF (2004) Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol 173:1978–1986
Rota P, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K (1990) Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 175:59–68
Sasaki S, Holmes TH, Albrecht RA, Garcia-Sastre A, Dekker CL, He XS, Greenberg HB (2014) Distinct cross-reactive B-cell responses to live attenuated and inactivated influenza vaccines. JID. doi:10.1093/infdis/jiu190
Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A (2013) Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 19:1305–1312
Suguitan AL Jr, McAuliffe J, Mills KL, Jin H, Duke G, Lu B, Luke CJ, Murphy B, Swayne DE, Kemble G, Subbarao K (2006) Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med 3:e360
Talaat KR, Karron RA, Callahan KA, Luke CJ, DiLorenzo SC, Chen GL, Lamirande EW, Jin H, Coelingh KL, Murphy BR, Kemble G, Subbarao K (2009) A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a Phase I trial in healthy adults. Vaccine 27:3744–3753
Talaat KR, Karron RA, Liang PH, McMahon BA, Luke CJ, Thumar B, Chen GL, Min JY, Lamirande EW, Jin H, Coelingh KL, Kemble GW, Subbarao K (2012) An open-label phase I trial of a live attenuated H2N2 influenza virus vaccine in healthy adults. Influenza Other Respir Viruses 7:66–73
Talaat KR, Karron RA, Luke CJ, Thumar B, McMahon BA, Chen GL, Lamirande EW, Jin H, Coelingh KL, Kemble G, Subbarao K (2011) An open label Phase I trial of a live attenuated H6N1 influenza virus vaccine in healthy adults. Vaccine 29:3144–3148
Talaat KR, Luke CJ, Khurana J et al (2014) A live attenuated H5N1 vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis 209(12):1860–1869. doi:10.1093/infdis/jiu123
Tam JS, Capeding MR, Lum LC, Chotpitayasunondh T, Jiang Z, Huang LM, Lee BW, Qian Y, Samakoses R, Lolekha S, Rajamohanan KP, Narayanan SN, Kirubakaran C, Rappaport R, Razmpour A, Gruber WC, Forrest BD, Pan-Asian C-TPETN (2007) Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatr Infect Dis J 26:619–628
Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K (2003) Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186
Toback SL, Ambrose CS, Eaton A, Hansen J, Aukes L, Lewis N, Wu X, Baxter R (2013) A postlicensure evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in children 24–59 months of age. Vaccine 31:1812–1818
Tomoda T, Morita H, Kurashige T, Maassab HF (1995) Prevention of influenza by the intranasal administration of cold-recombinant, live-attenuated influenza virus vaccine: importance of interferon-gamma production and local IgA response. Vaccine 13:185–190
Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, Singh S, Kinnersley N, Ward P, Mills RG (2000) Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 283:1016–1024
Treanor JJ, Kotloff K, Betts RF, Belshe R, Newman F, Iacuzio D, Wittes J, Bryant M (1999) Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 18:899–906
Vesikari T, Karvonen A, Korhonen T, Edelman K, Vainionpaa R, Salmi A, Saville MK, Cho I, Razmpour A, Rappaport R, O’Neill R, Georgiu A, Gruber W, Mendelman PM, Forrest B (2006) A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 25:590–595
Zangwill KM (2003) Cold-adapted, live attenuated intranasal influenza virus vaccine. Pediatr Infect Dis J 22:273–274
Zhu W, Higgs BW, Morehouse C, Streicher K, Ambrose CS, Woo J, Kemble GW, Jallal B, Yao Y (2010) A whole genome transcriptional analysis of the early immune response induced by live attenuated and inactivated influenza vaccines in young children. Vaccine 28:2865–2876
Acknowledgments
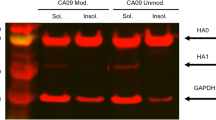
The seasonal influenza vaccine seed development program was conducted by the strain variant team of MedImmune at California. The pandemic vaccine program was conducted under a Cooperative Research and Development Agreement between MedImmune and NIH and was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH. We thank Dr. Zhongying Chen for providing data for Fig. 2 and Drs. Raburn Mallory and Kathy Coelingh for suggestions and comments.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Jin, H., Subbarao, K. (2014). Live Attenuated Influenza Vaccine. In: Oldstone, M., Compans, R. (eds) Influenza Pathogenesis and Control - Volume II. Current Topics in Microbiology and Immunology, vol 386. Springer, Cham. https://doi.org/10.1007/82_2014_410
Download citation
DOI: https://doi.org/10.1007/82_2014_410
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11157-5
Online ISBN: 978-3-319-11158-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)